Juvenile idiopathic arthritis – a new outlook

Juvenile idiopathic arthritis is the most common paediatric rheumatic disease, and can cause long-term joint damage or vision loss due to uncontrolled inflammatory uveitis. The outcomes for patients with juvenile idiopathic arthritis have significantly improved recently with advances in pharmacotherapy.
- Juvenile idiopathic arthritis (JIA) affects one to four in 1000 Australian children.
- Careful history and thorough clinical examination are essential in detecting and confirming arthritis.
- There is no diagnostic marker or antibody test for JIA; it is a diagnosis of exclusion.
- Early referral of the patient to a paediatric rheumatologist will facilitate early effective treatment.
- Biologic agents are increasingly used for children with JIA, with an excellent safety and efficacy profile.
Juvenile idiopathic arthritis (JIA) is not a rare disease. It is the most common paediatric rheumatic condition, affecting one to four in 1000 Australian children. Manifesting as persistent joint inflammation lasting at least six weeks, JIA is an umbrella term for seven different subtypes. In addition to arthritis, each subtype presents with distinct clinical features and variable outcomes. Although there is no known cure, management of JIA has been revolutionised in the past decade with the use of biologic drugs and thus sustained remission is an achievable goal.
This article provides GPs with a review of JIA’s clinical manifestations and diagnostic pitfalls, and an update on the latest management options. By maintaining awareness of this important condition, GPs can ensure timely referral to paediatric rheumatologists and facilitate the comprehensive care of children affected by JIA.
Clinical manifestations of JIA
Presentation
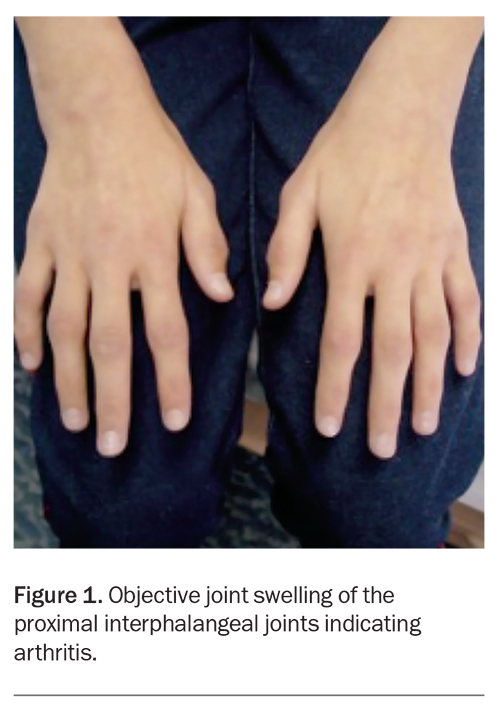
Understanding the distinction between joint pain (arthralgia) and joint inflammation (arthritis) is crucial before proceeding with further diagnostic evaluation. The common finding in all JIA subtypes is arthritis, which presents as objective and persistent joint swelling (Figure 1). There is typically a history of pain and stiffness in the affected joint(s), which is worse after rest and improves after activity or responds well to anti-inflammatory medication. The history may be prolonged, as JIA often has an insidious onset. A family history may also be relevant for autoimmune conditions, such as rheumatoid arthritis, psoriasis, ankylosing spondylitis or coeliac disease.
On examination, the affected joint may show a fullness and loss of contours compared with the contralateral side; although, this may be difficult to appreciate if there is bilateral swelling. The range of movement may be restricted and the joint may exhibit pain on movement. It is important to carefully examine all joints, particularly those above and below the reported painful joints as children may have difficulty localising symptoms. There may be atrophy of the muscles adjacent to the arthritic joint, due to disuse of that limb. Of note, the hip does not demonstrate joint swelling, but there may be decreased range of movement or a limp.
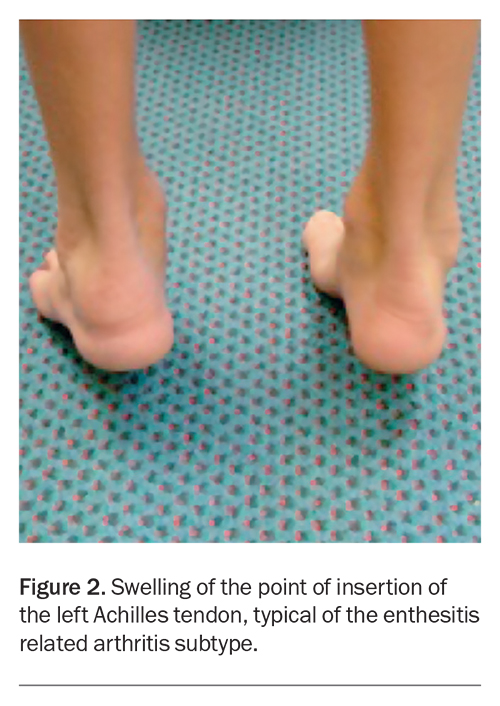
Although JIA can affect any joint, the knee, ankle, wrist and the small joints of the hand are commonly involved. A history of lower back pain with an inflammatory rhythm (e.g. early morning stiffness), particularly in an adolescent male, may be suggestive of spondylitis. This is a feature of the enthesitis-related arthritis subtype of JIA and, as the name suggests, those with this subtype of arthritis may also complain of pain at the insertion points of tendons (entheses) such as the Achilles tendon at the heel (Figure 2).
Extra-articular manifestations
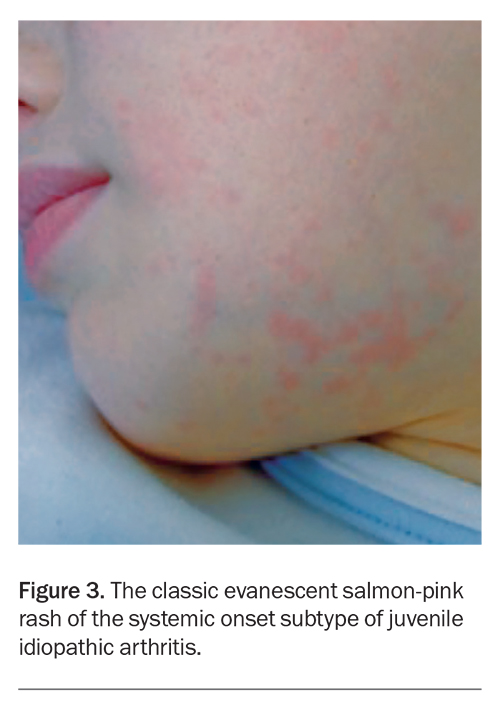
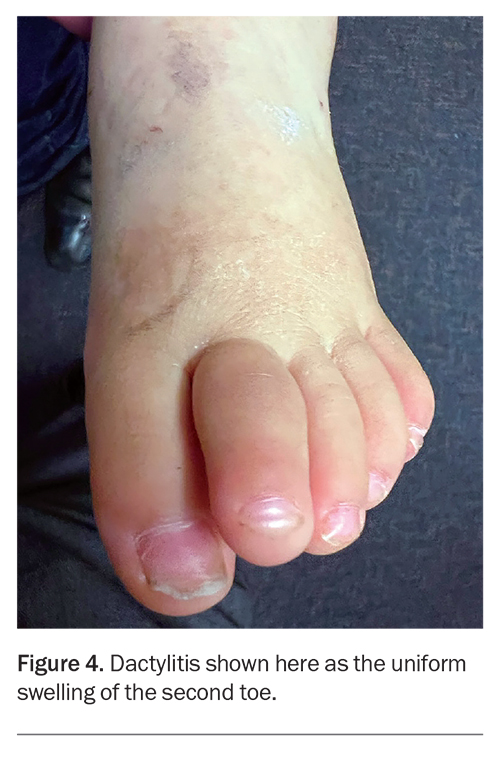
Extra-articular manifestations of JIA can also occur. The systemic onset subtype of JIA exhibits systemic features, such as spiking fevers, the classic evanescent salmon-pink rash (Figure 3), lymphadenopathy or serositis. Uveitis can also occur in patients with JIA, particularly in those with the oligoarticular subtype, and can manifest without symptoms. Symptoms of uveitis include eye redness, pain, photophobia and blurred vision. Uveitis can also manifest with no symptoms, therefore, vigilant screening is essential for early detection. Psoriatic changes (typical rash, nail bed pitting) or dactylitis (uniform swelling of a digit) may herald the psoriatic subtype of JIA (Figure 4).
Diagnostic pitfalls
A thorough understanding of the differential diagnoses is crucial as several conditions can mimic the clinical manifestations of JIA, which is ultimately a diagnosis of exclusion. The six-week timeframe before JIA can be appropriately diagnosed allows transient conditions such as viral-associated arthritis to resolve and other differential diagnoses to declare themselves (Box).
Infection
A monoarthritis should be considered a septic joint until proven otherwise, particularly if there is a short history, exquisite tenderness on movement or a history of fevers. Localised bony tenderness, warmth or erythema may reflect osteomyelitis. If either condition is suspected, the child should be urgently referred to a paediatric emergency department.
A history of a recent viral illness may suggest a postinfectious arthritis, which should resolve within the six-week time frame required for a diagnosis of JIA.
Poststreptococcal reactive arthritis may develop after streptococcal pharyngitis or impetigo. It is more common than rheumatic fever in the Caucasian population, with rheumatic fever being largely confined to Aboriginal and Torres Strait Islander populations in the Australian setting.
Malignancy
Malignancies such as leukaemia can manifest with bone pain that mimics arthritis. Patients should be assessed for weight loss, growth abnormalities, night sweats, and lymphadenopathy.
Other rheumatic conditions
Arthritis can also be a feature of rheumatic disease other than JIA. Systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis (e.g. Henoch-Schönlein purpura) can all present with joint involvement along with systemic symptoms. Features such as malar rash, photosensitivity, oral ulcers, myositis, Raynaud’s phenomenon, palpable purpura or constitutional symptoms should raise suspicion of these conditions.
Joint pain without joint inflammation
Joint pain alone, without objective evidence of joint inflammation (e.g. swelling, restriction in movement, warmth), should arouse suspicion of noninflammatory conditions. Biomechanical causes of joint pain, such as anterior knee pain of adolescence (patellofemoral syndrome), are typically exacerbated by exercise or activity. Chronic or complex pain syndromes, or hypermobility joint syndromes, frequently present with joint pains in the absence of persistent joint swelling (i.e. arthralgia rather than arthritis).
Investigations
Vigilance is required to differentiate JIA from alternative diagnoses and certain investigations can help exclude these confounding conditions. If the history and detailed physical examination still leave the diagnosis in doubt, it is important to remember that JIA itself has no diagnostic markers.
Blood tests
Full blood count (FBC) with low platelet count, low white cell count and anaemia should raise concern for a malignancy, whereas raised neutrophils and platelets suggest active inflammatory or septic arthritis. Although JIA may have an inflammatory pattern on FBC, the diagnosis is not excluded by a normal FBC.
Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate) can be useful if considering septic arthritis. They may be normal or only modestly elevated in most JIA subtypes, excluding those with systemic onset.
Lactate dehydrogenase level should be measured and a blood film ordered in cases of possible malignancy. Blood cultures are required if there are concerns for a concurrent infection or septic arthritis. Streptococcal titres (antistreptolysin-O and anti-DNAse B tests) are indicated if postinfectious arthritis is suspected.
Autoantibody screening
Rheumatological autoantibody screening should only be considered in those with clear evidence of inflammatory disease, such as arthritis or a characteristic rash. Screening in the absence of clinical signs often results in false negatives and false positives, leaving the ordering clinician with more questions than answers.
Antinuclear antibody (ANA) and extractable nuclear antigen tests are useful if systemic autoimmune conditions (e.g. systemic lupus erythematosus) are the probable cause, in combination with a clinical assessment that supports the diagnosis. They should not be ordered to investigate arthralgia in isolation, as a positive ANA test can occur in up to 20% of the population with no diagnostic relevance. An ANA test helps define the risk of uveitis in a child already diagnosed with JIA, but a negative ANA test does not preclude a diagnosis of JIA or uveitis.
Rheumatoid factor (RF) levels and anticyclic citrullinated peptide antibody levels can help clarify the subtype of JIA in children who have arthritis.
Presence of the HLA-B27 gene is associated with spondyloarthropathies and the enthesitis-related arthritis subtype of JIA. As it is present in 10% of the general population, HLA-B27 genetic testing is not suitable for diagnosis.
Joint aspiration
Joint aspiration and culture of synovial fluid is mandatory if there is a suspicion of septic arthritis.
Imaging
Plain x-rays are helpful in examining for biomechanical causes of joint pain. Ultrasound can objectively demonstrate joint inflammation, by revealing effusions, synovial hypertrophy or enthesitis. Ultrasound is particularly useful to examine the hip joints. An MRI is indicated if there is high suspicion for sacroiliac or temporomandibular joint inflammation.
Subtypes of JIA
The diagnosis of JIA will often be made clinically by a paediatric rheumatologist. The subtype is further characterised based on the results of pathology tests and imaging.
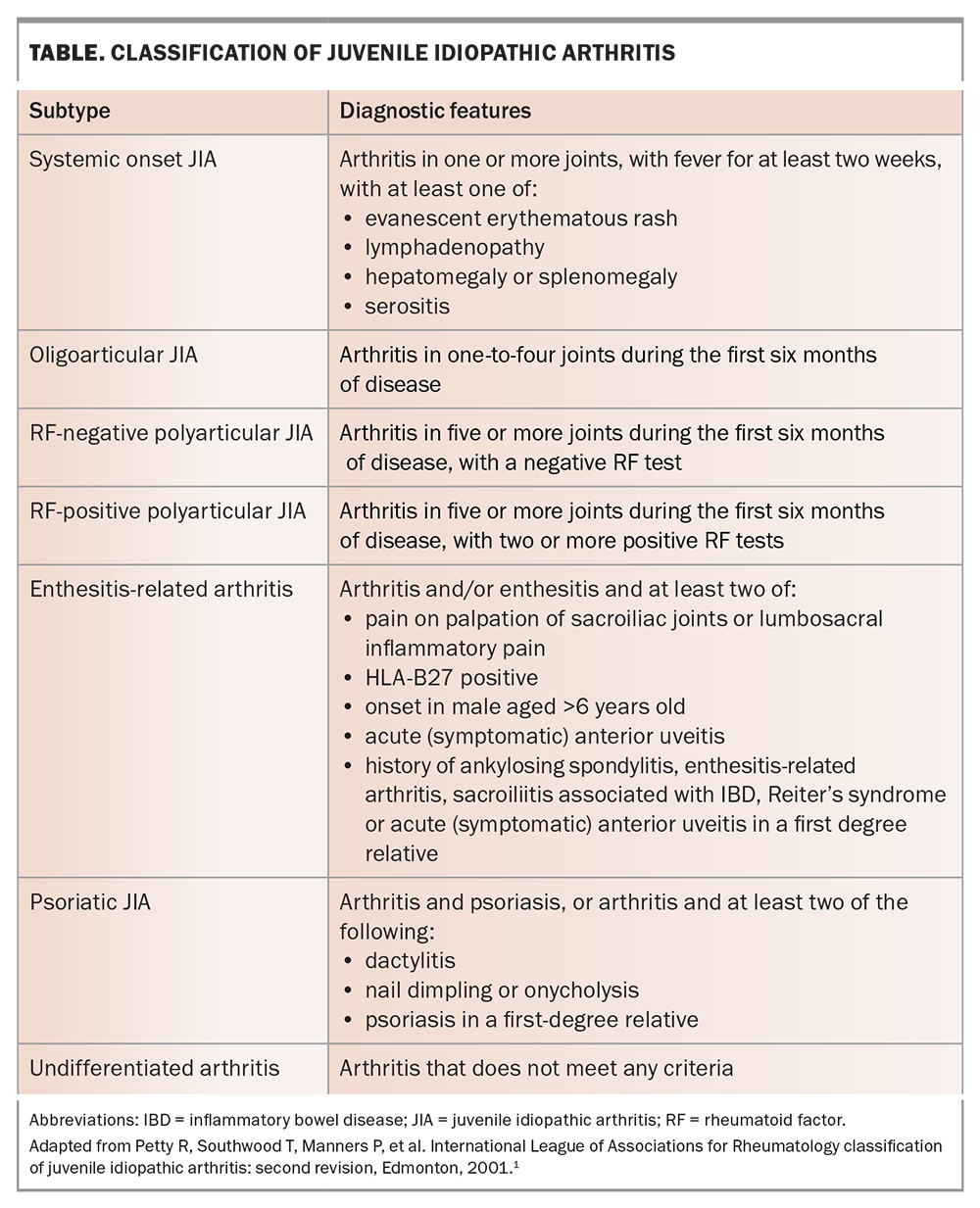
The International League Against Rheumatism (ILAR) criteria are used for classification in both clinical and research settings.1 There are seven subtypes of JIA: oligoarticular JIA, RF-positive polyarticular JIA, RF-negative polyarticular JIA, enthesitis-related arthritis, systemic onset JIA, psoriatic arthritis and an undifferentiated form for those who do not fit the above criteria. Each subtype has its own clinical manifestations, as outlined in the Table.
There is much debate regarding the ILAR criteria, with modifications expected soon. For example, it is increasingly accepted that RF or anticyclic citrullinated peptide antibody positive polyarthritis is the same condition in children as it is in adults (i.e. rheumatoid arthritis), whereas enthesitis-related arthritis is the clinical correlate of ankylosing spondylitis, and systemic onset JIA has the same pathophysiological process as adult onset Still’s disease.
Management
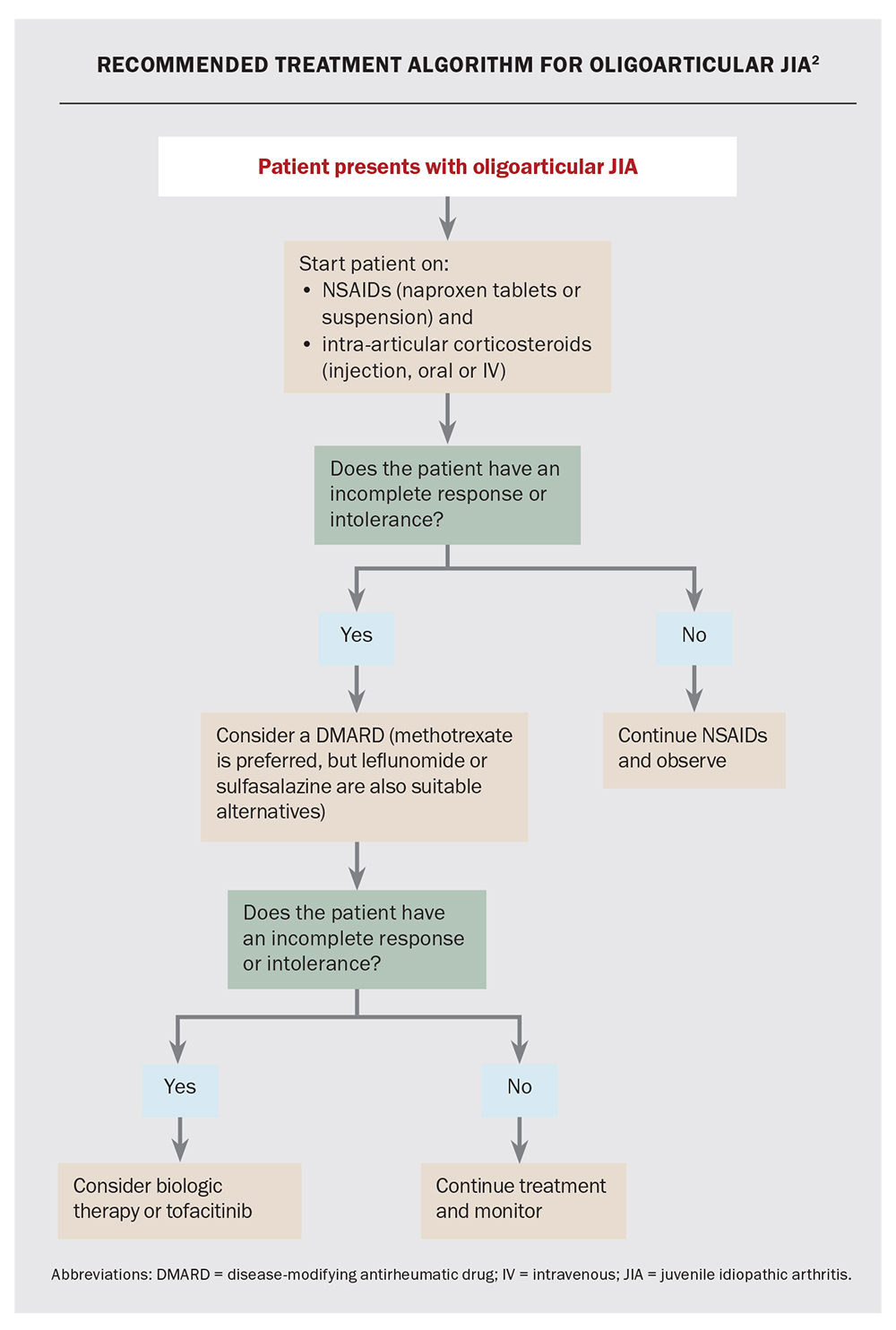
Management of JIA should be guided by a paediatric rheumatologist and individualised to both the patient and the subtype (Flowchart). Additionally, GPs play a crucial role as the primary point of contact for patients and their families.
NSAIDs
NSAIDs remain first-line therapy for children with JIA to alleviate pain, reduce inflammation and improve joint function. GPs can prescribe NSAIDs to help manage JIA associated pain, while the patient awaits review by a paediatric rheumatologist. Although effective for analgesia, NSAIDs alone do not alter the progression of underlying disease and do not prevent chronic joint damage.
Naproxen is often used for JIA because of its long half-life, convenient twice-a-day dosing schedule and useful suspension form. Naproxen tablets can be prescribed via a standard PBS prescription, whereas the suspension form is authority required. However, other NSAIDs may be used and should be considered based on patient and clinician preference. Patients and their families should be reminded to only use one NSAID at a time, including any over-the-counter NSAIDs (e.g. ibuprofen). If required, paracetamol can be used for additional analgesia.
GPs should monitor the use of NSAIDs by evaluating their effectiveness in managing symptoms and ensuring the patient is receiving an appropriate dose. The most common side effect is gastritis and microscopic blood loss; rarely, renal dysfunction and hepatotoxicity can occur with prolonged use.
Corticosteroids
Most subtypes of JIA are initially managed with NSAIDS and intra-articular corticosteroid injections, which involve injecting a long-acting preparation into the affected joint(s). This often leads to quick resolution of arthritis in that joint, which is sustained for at least six months (often much longer). Although the procedure itself is quick and safe, most children will require sedation or a general anaesthetic.
Oral or intravenous corticosteroids may be used in select cases, particularly in systemic onset JIA, but this should occur only after careful exclusion of the differential diagnoses by the treating paediatric rheumatologist. GPs should not prescribe oral corticosteroids for patients with undifferentiated arthritis.
Disease-modifying antirheumatic drugs
The paediatric rheumatologist may choose to start a disease-modifying antirheumatic drug (DMARD) in severe cases or if corticosteroid joint injections have proved ineffective. Methotrexate is the commonly used nonbiologic DMARD, with leflunomide or sulfasalazine as suitable alternatives. Methotrexate can be administered either orally or subcutaneously and is given once per week. Although generally very well tolerated, the most common adverse effect of methotrexate is nausea and vomiting occurring within 24 hours of administration. Oral ulcers may also occur. Folic acid is routinely given to help reduce these side effects. Regular monitoring of blood counts, liver function and renal function is necessary to assess for potential adverse effects.
Based on adult rheumatoid arthritis data, patients on methotrexate are slightly more vulnerable to infection. Children on methotrexate who are exposed to varicella zoster infection require specific antiviral therapy and will often be warned of this by their paediatric rheumatologist.
Biologic therapy
Biologics have transformed the treatment of many autoimmune conditions, including JIA. They are generally well tolerated with good safety profiles. The paediatric rheumatologist may choose to introduce a biologic agent, depending on a patient’s progress on corticosteroids or DMARDs.
Adalimumab is a biologic specifically targeting tumour necrosis factor-alpha and is currently the first-line biologic agent in Australia to treat polyarticular JIA that has failed to respond to inadequate response to one or more disease DMARDs. It is given via a subcutaneous injection every two weeks and has proven very effective in inducing and maintaining long-term remission in JIA. It is also effective in patients with uveitis. Adverse effects include injection site reactions and a slightly increased susceptibility to infections; however, the incidence of serious adverse events is low.
Other commonly used biologic agents for JIA include other tumour necrosis factor-alpha inhibitors (e.g. infliximab [off-label use] and etanercept), the interleukin-6 inhibitor, tocilizumab, or, in cases of systemic JIA, the interleukin-1 inhibitor anakinra. Canakinumab is another interleukin-1 inhibitor; however, is very costly and usually difficult to access and, therefore, not typically used in the treatment of JIA. Abatacept is indicated for paediatric patients aged 6 years and older, with moderately to severely active polyarticular JIA who have had an inadequate response to one or more DMARDs. Usually, these medications are only weaned after prolonged remission.
JAK inhibitors – targeted synthetic DMARDs
Tofacitinib, a Janus kinase (JAK) inhibitor, was approved by the TGA in January 2023 for active polyarticular JIA and juvenile psoriatic arthritis in patients aged 2 years and older, who have previously responded inadequately to DMARDs. It is anticipated to be PBS listed for JIA in the coming months. A recent double-blind, placebo-blinded, withdrawal phase 3 trial of tofacitinib for polyarticular JIA found statistically significant lower flare rates in tofacitinib users versus placebo.3 In contrast to biologics, which are delivered via subcutaneous or intravenous route, JAK inhibitors are orally administered (an important consideration for children).
Other management considerations
With increased treatment options, most children with JIA in Australia are expected to be in remission within 12 months of diagnosis; however, vigilance is required as relapses are common.4 Beyond medication monitoring, GPs should emphasise the importance of regular physical activity, including stretching and strengthening exercises to help maintain joint function. Referring patients to physical and occupational therapists can provide additional support and guidance. A well-balanced, nutritious, and healthy diet is recommended, although no specific foovds or dietary restrictions are shown to be of benefit.5
GPs should always consider the psychosocial burden of JIA and its potential effects on mental health. Collaborating with psychologists or counsellors can assist in addressing emotional challenges and enhancing overall wellbeing. Finally, uveitis is a sight-threatening complication of JIA and the paediatric rheumatologist will typically refer most patients for routine ophthalmology screening.
Conclusion
JIA encompasses a heterogeneous group of conditions, with joint inflammation being the unifying feature. Thorough clinical assessment, systematic exclusion of differential diagnoses (e.g. infection or malignancy) and referral to a paediatric rheumatologist are key determinants of early diagnosis and optimal outcome. Management options are continually improving, promising an even brighter future for children with JIA. MT
COMPETING INTERESTS: Dr Sinnappurajar: None. Dr Chaitow holds consultancy positions with Pfizer and Novartis.
Further reading
Petty R, Laxer R, Lindsley C, et al. Juvenile idiopathic arthritis: classification and basic concepts. 8th ed. Elsevier; 2021.
ANZMUSC. An Australian living guideline for the management of juvenile idiopathic arthritis. 2022 [version 0.2]. Available from: https://app.magicapp.org/ (accessed October 2023).
References
1. Petty R, Southwood T, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31: 390–392.
2. Onel KB, Horton DB, Lovell DJ, et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Care Res 2022; 74: 521-537.
3. Ruperto N, Brunner HI, Synoverska O, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet 2021; 398: 1984-1996.
4. Tiller G, Buckle J, Allen R, et al. Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of an inception cohort of Australian children. Pediatr Rheumatol 2018; 16: 69.
5. Onel KB, Horton DB, Lovell DJ, et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: recommendations for nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Arthritis Care Res 2022; 74: 505-520.
Acknowledgement
Dr Sinnappurajar’s clinical training is funded by the Arthritis Australia Paediatric Rheumatology Advanced Training Scholarship.