An update on travel vaccinations

Many travellers visiting low-income countries, or travelling in groups or on cruise ships, will experience a travel-associated ailment. Therefore, a personalised pretravel health consultation is essential to assess the risks for an individual traveller and to offer appropriate advice and vaccinations. In some instances, vaccinations will be required for crossing international borders.
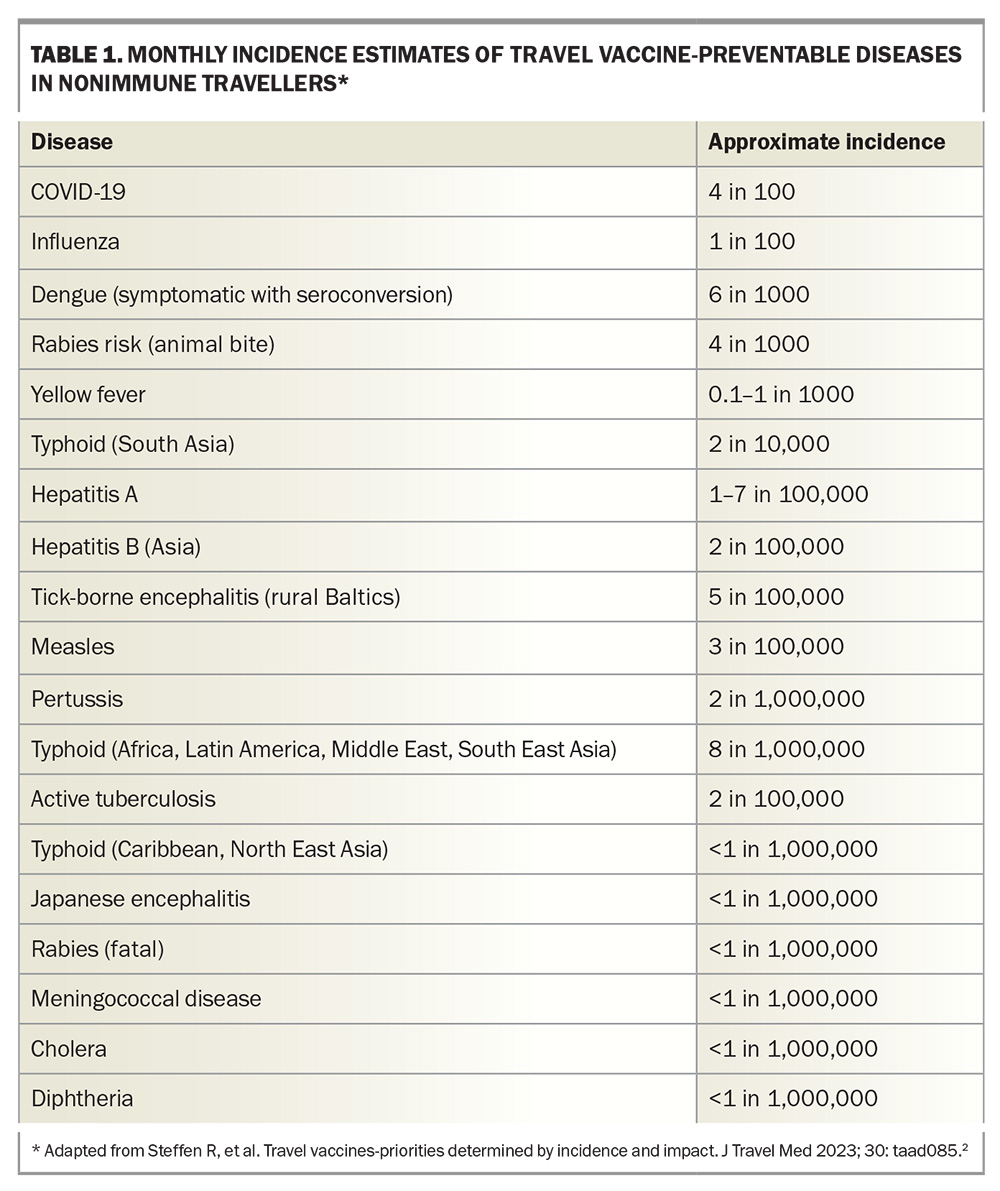
As travellers are able to cross borders and visit all points of the globe, they can be exposed to various hazards. It has been estimated that up to half of all travellers visiting low-income countries for one month will experience some travel-associated ailment.1 Recently updated estimates of monthly incidence rates of vaccine-preventable diseases in nonimmune travellers show that COVID-19 is now the most common of these diseases, followed by influenza, which affects 1% of travellers per month (Table 1).2 This highlights the importance of discussing the potential benefits of COVID-19 and influenza vaccination with patients who are intending to travel, especially those travelling in groups or on cruise ships.
Illness among travellers depends on a variety of factors that the health practitioner needs to ascertain during the pretravel consultation. To provide appropriate travel health advice, practitioners must obtain sufficient information from the traveller to enable an adequate personalised risk assessment. A complete risk assessment should cover the individual, means of travel, destination and, ultimately, any intervention that will be used (Box 1).
After the risk assessment has been completed, the pretravel health consultation generally consists of three main areas: vaccination, education and prescribing chemoprophylaxis and travellers’ medical kits.
Travel vaccinations can be divided into three groups:
- routine vaccinations
- required vaccinations
- recommended vaccinations according to risk of disease.
This article provides an update on the vaccinations that are currently available in Australia and relevant to the pretravel consultation.
Routine childhood or adult vaccinations
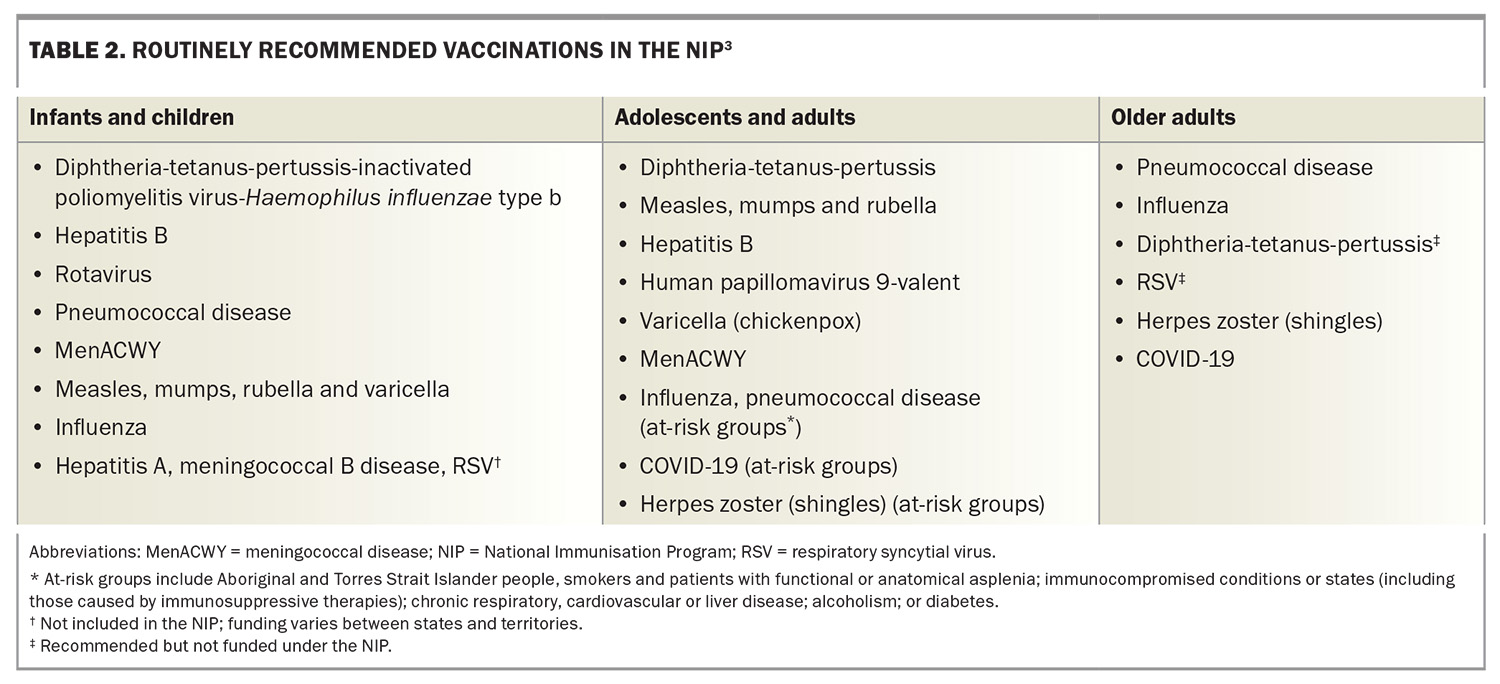
It is important to check that all travellers are up to date with their routine vaccinations appropriate for their age, underlying health conditions, occupation and lifestyle, according to the National Immunisation Program (NIP) (Table 2). If not, these should be updated, as the risk of exposure to routine vaccine-preventable diseases is higher than the risk of exposure to more exotic or tropical infections, such as tick-borne encephalitis or typhoid, during international travel.
Relevant routine vaccinations include those for hepatitis B and measles, mumps and rubella (MMR) in young adults and for influenza in all travellers, especially young children, at-risk groups (e.g. people with specific medical conditions) and older people travelling in large tourist groups or on cruise ships. All infants and children aged younger than 2 years are also strongly recommended to receive vaccination against meningococcal B disease.3
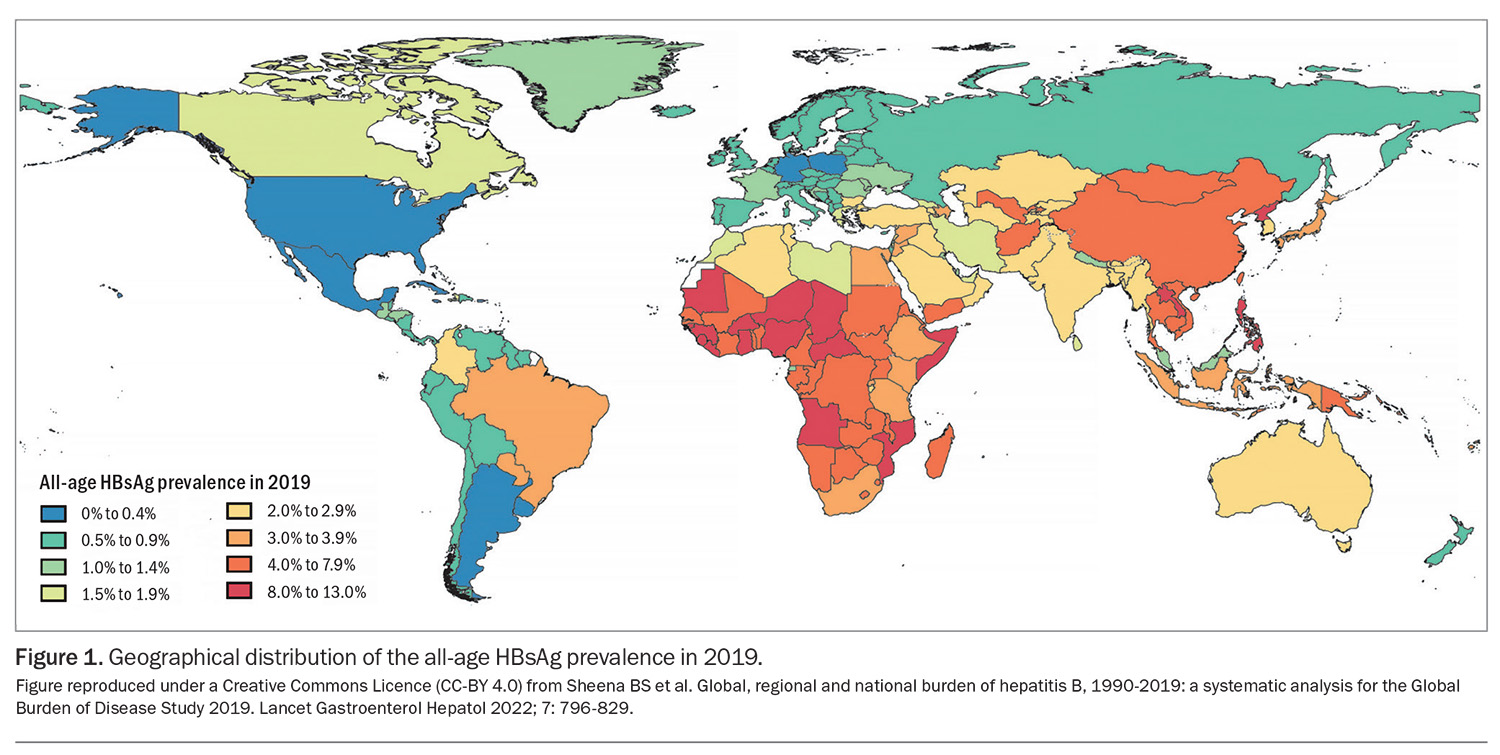
Most Australian children born in 2000 or later have been vaccinated against hepatitis B under the NIP. Hepatitis B vaccination is recommended for people undertaking long-term (more than four weeks) or frequent travel to regions of high or intermediate hepatitis B endemicity (Figure 1), including Central and South America, Africa, Asia and certain destinations in Oceania.3 As well as the region of travel, the behaviour of travellers while overseas is important in determining their individual risk of hepatitis B exposure. Travellers may be exposed to the virus through bloodborne (including during emergency or planned medical or dental procedures) or sexual routes.4
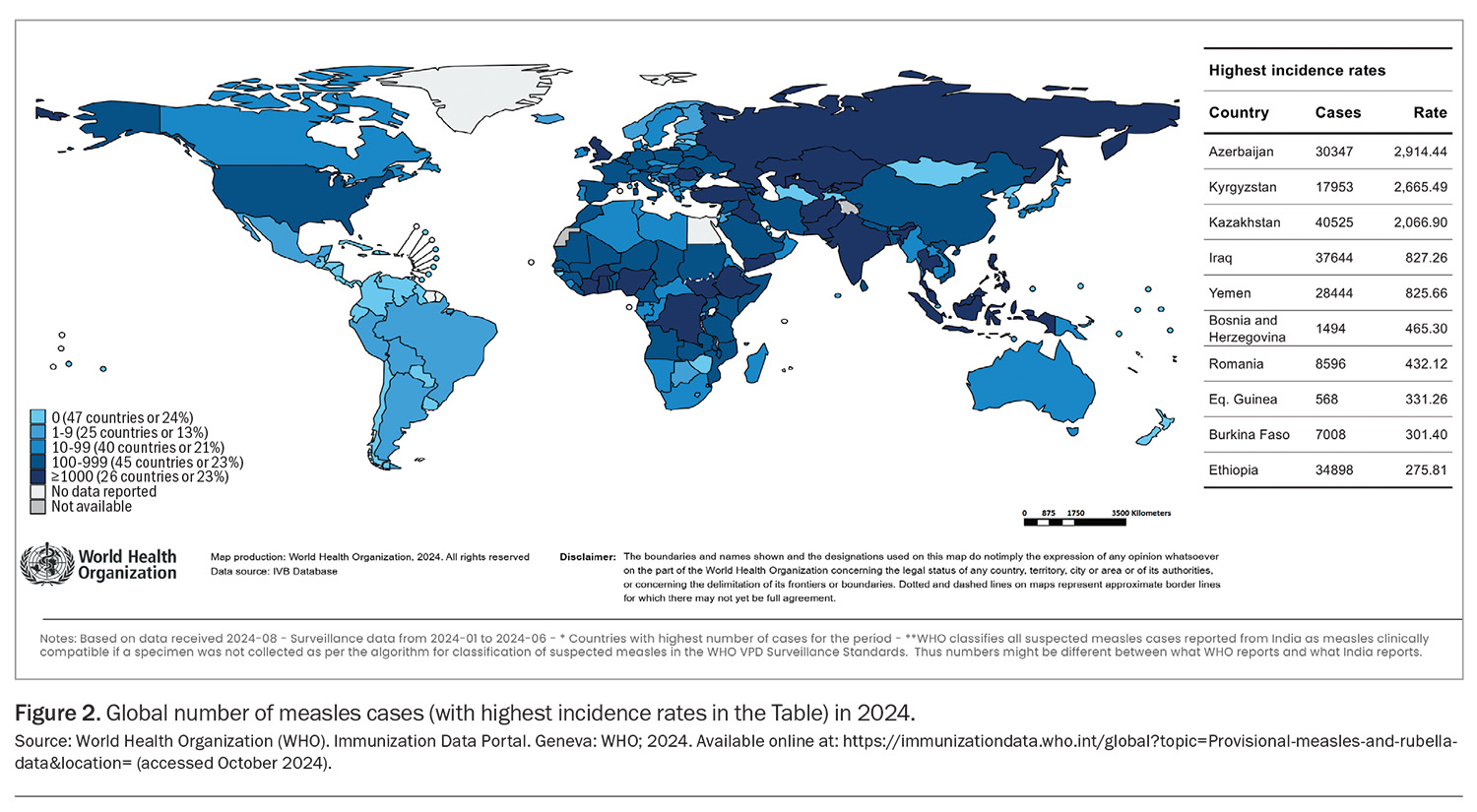
There are ongoing outbreaks of measles and mumps in Europe, the Indian subcontinent, Asia, Africa and the Americas (Figure 2), with most outbreaks in Australia being traced back to infected returning travellers who were inadequately vaccinated.3 Overseas travellers born in 1966 or later are now recommended to have either documented evidence of two doses of measles- containing vaccine given at least four weeks apart or serological evidence of immunity to MMR.3
Further, infants aged younger than 12 months who are travelling to countries where measles is endemic or where measles outbreaks are occurring may receive an MMR vaccine from as young as 6 months of age, after an individual risk assessment. However, this dose needs to be repeated, meaning that these infants need two further doses of a measles-containing vaccine: at 12 months (or four weeks after their first dose, whichever is later) and again at 18 months of age, as per the NIP.3
Required vaccinations
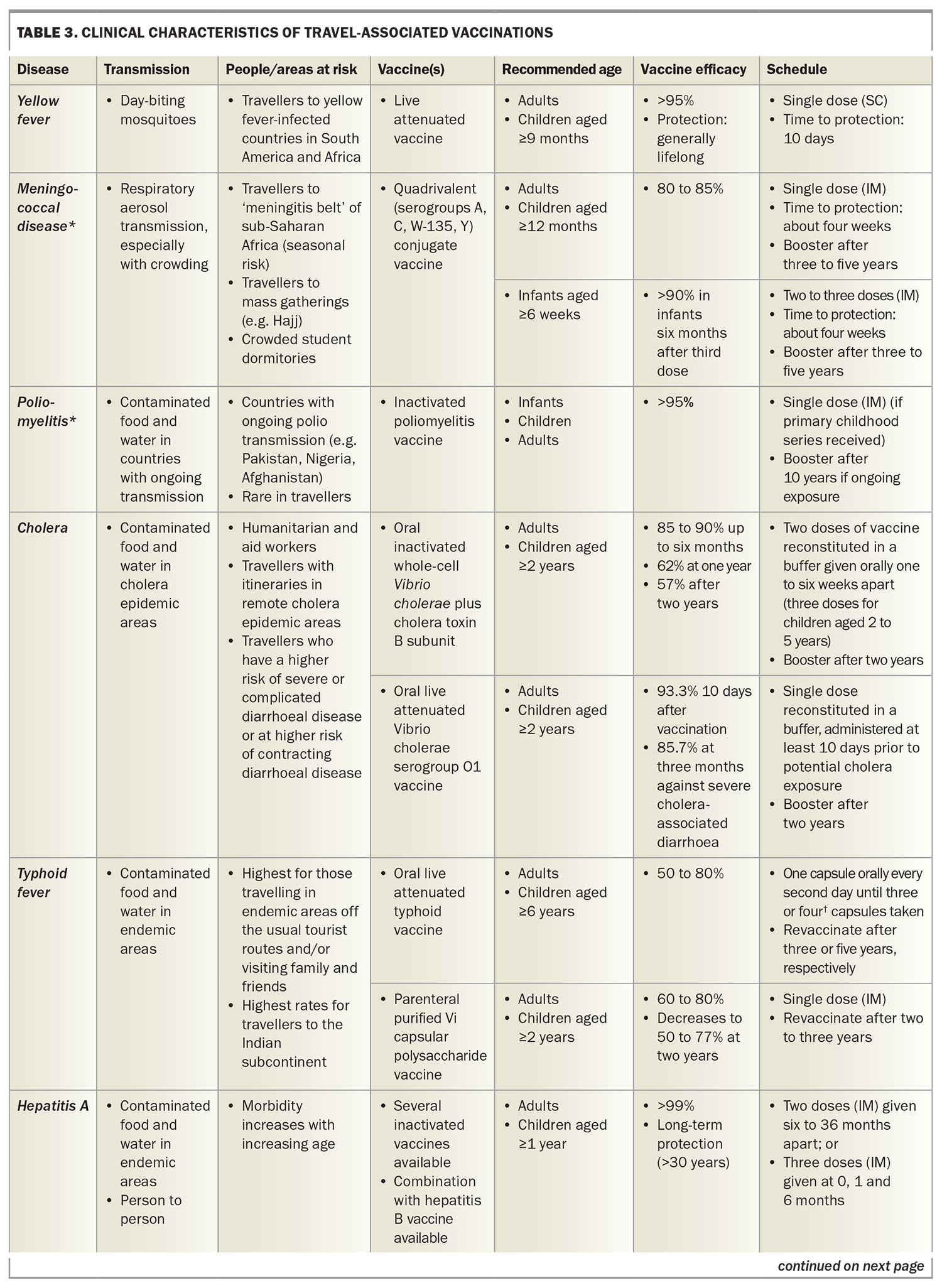
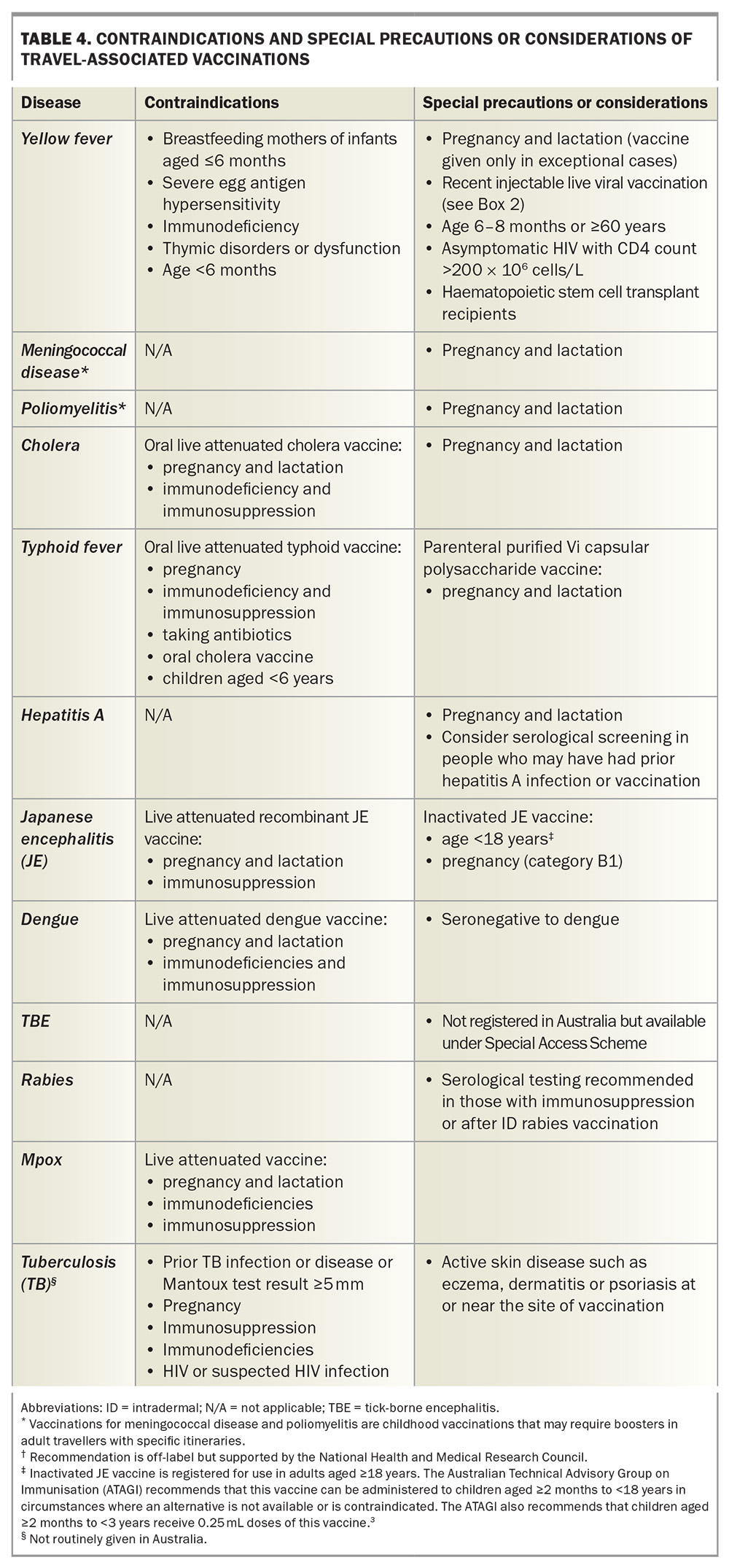
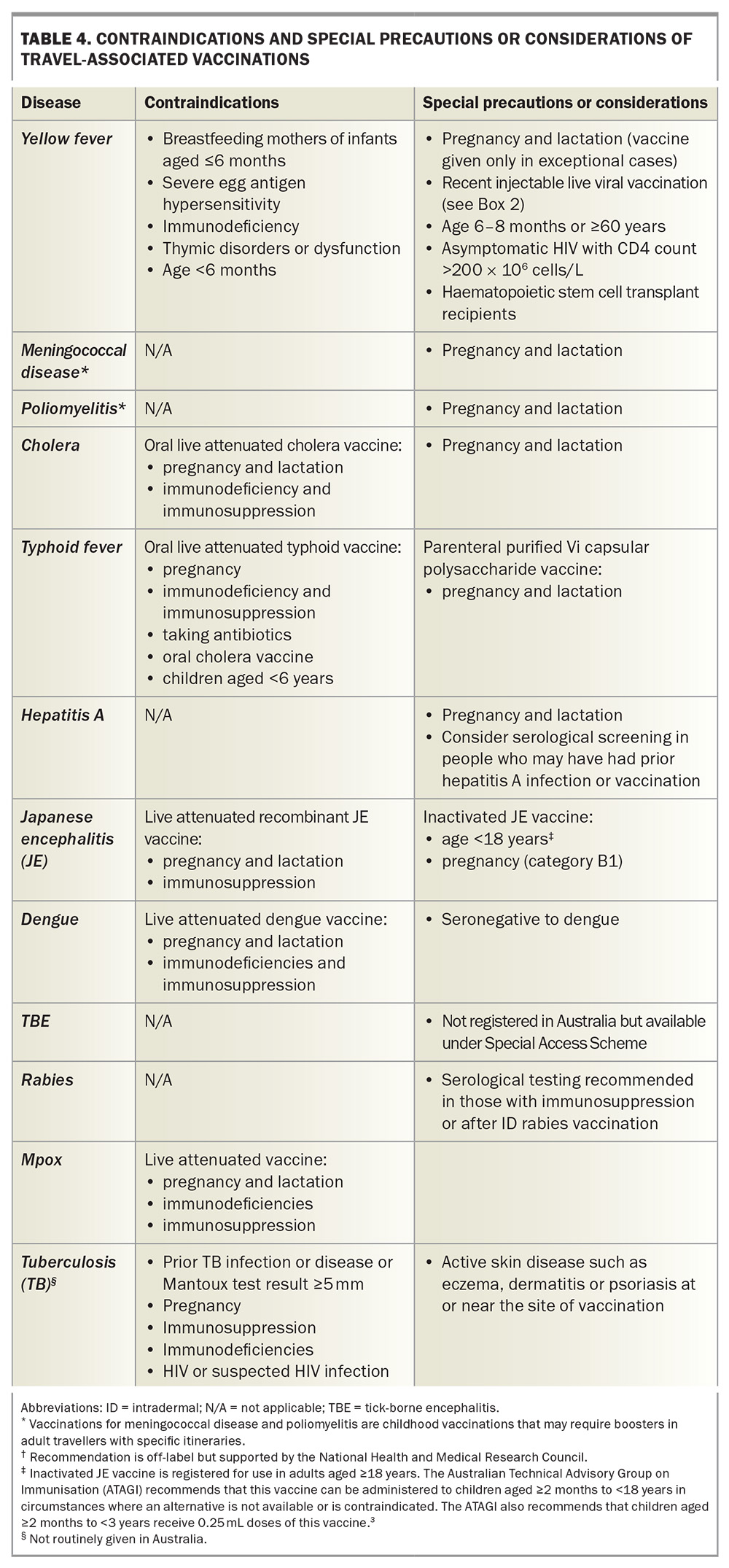
Details of travel-associated vaccines are summarised in Table 3. Contraindications, special precautions and considerations for these vaccines are listed in Table 4.
Yellow fever
The vaccine used for the prevention of yellow fever, which is a mosquito-borne illness, can only be administered by registered yellow fever vaccination providers. These vaccine providers have been approved by their relevant state or territory health authorities. The practitioner needs to determine whether the traveller will be at risk of yellow fever at the destination or whether vaccination is required under the International Health Regulations (IHR).5
Some countries have specific yellow fever vaccination requirements or changing requirements that are not explained by looking at the yellow fever maps.6 It is therefore important to understand the full details of the traveller’s itinerary, including countries that may be briefly transited through, to have up-to-date data on yellow fever transmission and to be aware of current vaccine recommendations.
As there are rare but significant adverse events associated with yellow fever vaccination (including an anaphylaxis rate of 1.3 per 100,000 doses administered and a rate of vaccine-associated neurotropic or viscerotropic events of one to two per 1,000,000 doses administered), the risk of these events must be balanced against the true risk of exposure to yellow fever. If, on balance, the traveller has a high risk of significant vaccine-associated adverse events (e.g. age older than 60 years) and is undertaking low-risk travel, an exemption or waiver certificate from the IHR requirements may need to be considered.7
As per Annex 7 of the IHR, a single dose of a yellow fever vaccine approved by the World Health Organization (WHO) is sufficient to confer sustained immunity and lifelong protection against yellow fever disease in most healthy adults and children.8 Although 10-yearly boosters are no longer required for international travellers, some data suggest that vaccination of pregnant women and people with lowered immunity, such as those with HIV infection, may not provide lasting protection. These groups therefore require a booster vaccination.8 It should be noted that almost all cases of yellow fever vaccine-associated neurotropic and viscerotropic events reported globally occured in first-time vaccine recipients, with no reported cases after booster doses.9
The International Certificate of Vaccination or Prophylaxis (ICVP) should be completed for the traveller. This certificate becomes valid 10 days after the day of vaccination and remains valid for life.
Meningococcal disease
The quadrivalent meningococcal (MenACWY) vaccine is included in the ‘required’ vaccination category because it is mandated by Saudi Arabian authorities for religious pilgrims, including those travelling for the Hajj and Umrah. Meningococcal meningitis is caused by the bacteria Neisseria meningitidis, which are transmitted from human to human via respiratory droplets, especially in crowded conditions. It is endemic in sub-Saharan Africa (the ‘meningitis belt’), which has the highest rates of the disease worldwide. During epidemics in this region, the incidence can approach 1000 per 100,000 people, or 1% of the population.7
Poliomyelitis
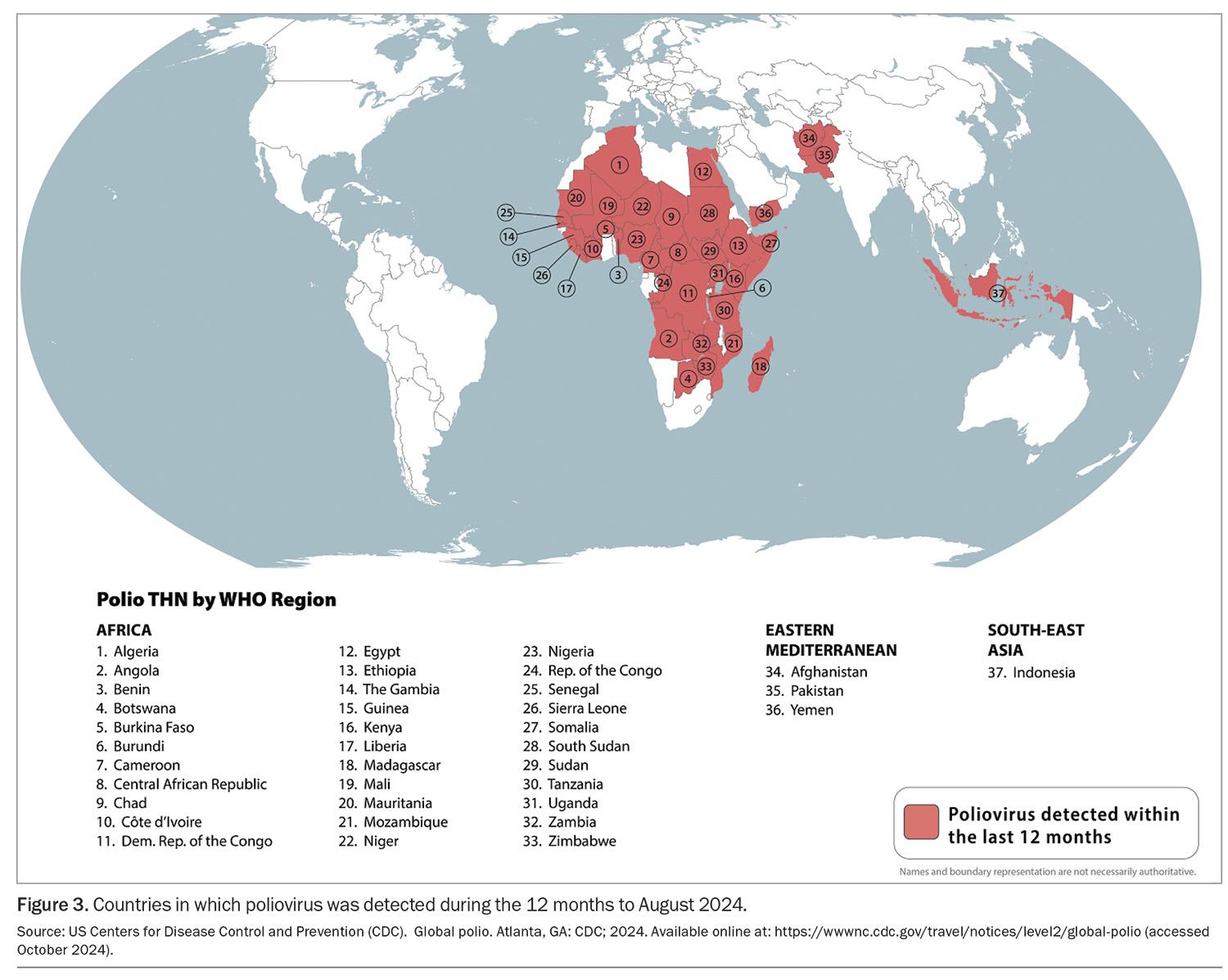
Poliomyelitis is rare in travellers. Before the COVID-19 pandemic, the Global Polio Eradication Initiative had decreased cases by more than 99%, with Afghanistan and Pakistan being the only two countries where poliomyelitis remained endemic.10 The pandemic disrupted vaccination campaigns, and over 30 countries are reporting circulating poliovirus currently (Figure 3).11 These include Indonesia, which recently overtook New Zealand as the most common overseas destination for Australians, according to 2023 Australian Bureau of Statistics data. Canada, the UK and the USA are no longer considered polio-infected but reported circulating vaccine-derived poliovirus as recently as 2022.11
International travellers should ensure they are fully vaccinated against polio before departure. Governments of countries with an increased risk of exposure to poliovirus may require visiting travellers to show proof of recent polio vaccination on their yellow ICVP when departing that country.12 Adult travellers should therefore be offered a one-off booster of inactivated poliomyelitis vaccine (given as IPV or dTpa-IPV if requiring additional dTpa booster concomitantly) if their destination has circulating poliovirus, they have completed their routine vaccination series and they have not already received an adult booster dose. Practitioners need to ensure that such travellers are provided with an ICVP in the form specified in Annex 6 of the IHR to serve as proof of vaccination.12
Recommended vaccinations
Recommended vaccinations include those for vaccine-preventable diseases transmitted through contaminated food and water, from person to person via the faecal–oral route (cholera, typhoid fever, hepatitis A and poliomyelitis), by vectors (Japanese encephalitis [JE], yellow fever, dengue and tick-borne encephalitis), infected animal bites (rabies) and respiratory droplets (influenza, meningococcal disease, COVID-19, respiratory syncytial virus infection and tuberculosis [TB]). Table 3 and Table 4 summarise the attributes, scheduling and contraindications for recommended vaccinations, which are prescribed according to the risk assessment undertaken for the traveller. As noted above, some recommended vaccinations are also required for crossing international borders.
Cholera
Cholera is rare in Australian travellers, as most do not visit areas with active cholera transmission, with three cases notified in each of 2022 and 2023 and two notified to date (September) in 2024.14
Cholera vaccination should be considered for humanitarian and aid workers and those with itineraries in remote cholera-epidemic areas. Cholera vaccination is also recommended for travellers who have a higher risk of severe or complicated diarrhoeal disease and those at high risk of contracting diarrhoeal disease, including people with poorly controlled or complicated diabetes, inflammatory bowel disease, HIV infection or other immunocompromising conditions, significant cardiovascular disease or primary or secondary achlorhydria.3
Two cholera vaccines are registered in Australia: a single-dose, oral live attenuated vaccine and a two-dose oral inactivated vaccine, with the second dose given one to six weeks after the first. These vaccines have similar protective efficacy (85 to 90%) against severe Vibrio cholera at three months postvaccination.
Typhoid
The risk of exposure to Salmonella enterica serotype Typhi is the highest for people travelling off the usual tourist routes and those visiting family and friends.7 The highest rates of the disease are found in those travelling to the Indian subcontinent.7 The estimated risk of contracting typhoid in travellers to South Asia is about 1 in 5000 people.2
Two typhoid vaccines are registered in Australia: an oral live attenuated vaccine and a parenteral (injectable) Vi polysaccharide antigen vaccine. These vaccines have similar protective efficacy (50 to 80%) against S. typhi, although the injectable vaccine provides no cross-protection against S. paratyphi B, unlike the oral vaccine.15S. paratyphi B is more common in South and Central America.
The oral typhoid vaccine strain may be destroyed by gastric acid, so the capsules must be swallowed whole rather than chewed or opened. There should be an interval of at least eight hours between administering the oral typhoid vaccine and the inactivated oral cholera vaccine, as the buffer in the cholera vaccine may affect transit of the typhoid vaccine capsules through the gastrointestinal tract. The oral typhoid vaccine may also be susceptible to inactivation by some antibiotics and antimalarial agents (including doxycycline). If the oral vaccine is used, it is recommended that vaccination be timed so that the last dose is administered at least three days before starting oral antibiotics. Unlike injectable live vaccines, the oral live typhoid vaccine does not interact with other injectable live vaccines, but its use is contraindicated in people who are immunocompromised or pregnant.
Hepatitis A
The risk of contracting hepatitis A infection has continuously reduced over the past four decades and is now less than one per 10,000 travellers per month, depending on the destination and style of travel in hepatitis A-endemic countries.2 Morbidity from this infection increases with age, with a case-fatality rate of 1.8% in people aged older than 50 years.7
Several inactivated hepatitis A vaccines are registered in Australia, all of which are highly efficacious, well tolerated and provide long-term protection (at least 30 years) in both children and adults, provided a complete course is given.2,8 The hepatitis A vaccine is also available in combination with the hepatitis B vaccine or typhoid vaccine, although the hepatitis A and typhoid vaccine combination will not be available in Australia after the existing stock expires in November 2024. Serological screening should be considered in people who may have had prior hepatitis A infection or illness.
Japanese encephalitis
JE is a rare viral disease that can infect the brain and cause potentially devastating complications. It is transmitted by mosquitoes that bite at dusk and/or dawn and occurs in Asia and parts of the western Pacific. Until recently, cases were detected in Australia only in the Torres Strait, Tiwi Islands and, rarely, in far north Queensland.16 In 2022, a widespread outbreak of JE occurred in mainland Australia in a previously nonendemic area, with 41 cases notified in that year, compared with eight cases over the previous three years.14 This was declared a Communicable Disease Incident of National Significance.
JE is generally viewed as an infection of agricultural areas (risk correlates with exposure in endemic areas). Most cases (more than 99%) of JE are mild or asymptomatic. The case-fatality rate is 20 to 30% in symptomatic individuals (up to 50,000 deaths are reported annually), with another 30 to 50% having severe neurological, cognitive or psychiatric sequelae.7,17 At least 75% of cases occur in children aged 14 years or younger, with an overall incidence of five to 50 cases per 100,000 children per year in Asian populations.7,17
The JE vaccination recommendations for people travelling to Asia and Papua New Guinea are customised according to the season of travel, regions to be visited (urban or rural), duration of travel and extent of outdoor activities.3 The VaxiCal risk-benefit calculator has been developed to help decision-making about JE vaccination in travellers for this low incidence but high morbidity and mortality disease (https://www.vaxical.com).18 State- and territory-funded JE programs are in place for high-risk groups (https://www.health.gov.au/diseases/japanese-encephalitis).
There are two JE vaccines registered in Australia: a two-dose vero-cell-derived inactivated vaccine, given at days 0 and 28; and a single-dose live attenuated recombinant vaccine. The two-dose inactivated vaccine schedule should be completed at least one week before exposure, whereas the live recombinant vaccine should be administered in adults at least two weeks before exposure. There is an age-dependent need for boosters of each vaccine.
A rapid two-dose schedule (seven days apart) for the inactivated vaccine is approved in Europe for adults aged 18 to 65 years if time does not allow for a four-week interval between doses, but this is not yet approved in Australia.17
Rabies
Rabies occurs in more than 150 countries and territories globally. The infection is almost universally fatal in humans, with 59,000 deaths occurring worldwide each year, mostly in Asia and Africa.19 The rabies virus is transmitted through bites and scratches from, or mucosal exposure to, infected animals: mostly dog bites, scratches or mucosal exposure, but also from bats, cats and monkeys. The rate of rabies exposure for travellers is at best an estimate and may range from 16 to 200 per 100,000 travellers.7 Of people who are bitten by suspected rabid animals, 40% are children aged younger than 15 years.20 Every year, more than 15 million people worldwide receive postexposure vaccination to prevent the disease.20
Pre-exposure prophylactic (PrEP) vaccination provides partial immunity against rabies virus infection. Studies of the rabies vaccines registered in Australia have shown that a two-dose PrEP schedule produces an immune response comparable with that produced by a three-dose PrEP schedule, particularly in the seven to 28 days after vaccination. The standard rabies PrEP course was therefore recently revised from three vaccine doses down to two doses given seven days apart, with the proviso that a third dose is given within 12 months of initial vaccination, either intramuscularly or intradermally (off-label use), especially if ongoing or repeat potential rabies exposure is anticipated.21 In the event of an exposure, two additional doses of vaccine, given three days apart, are still required.
The two-visit PrEP vaccine schedule will be particularly beneficial for last-minute travellers to rabies-enzootic areas. The two-visit PrEP schedule is not recommended for people who are immunocompromised as their immune response may not be adequate.
Intradermal administration of the rabies vaccine has not yet been approved by the TGA, so its use remains off-label, despite this route being endorsed by the WHO.19 If the intradermal route is being considered, the National Health and Medical Research Council (NHMRC) provides guidance on the factors that need to be considered.3
Rabies PrEP ensures the traveller receives safe, efficacious vaccination and simplifies postexposure management. Further, there is no lower age limit for vaccine administration. In the event of an animal bite, fewer doses of the vaccine will be required, with no need for rabies immunoglobulin, which is often expensive and difficult or impossible to obtain in many developing countries.
Routine serological testing for rabies after intramuscular PrEP vaccination is not usually necessary. However, people who are immunocompromised or who received intradermal PrEP vaccination should have their rabies virus neutralising antibody titres determined 14 to 21 days after the third dose of the vaccine, and a further dose should be given if the titre is reported as inadequate (i.e. less than 0.5 IU/mL). Serological testing should then be repeated and, if titres remain below 0.5 IU/mL, expert advice should be sought.3
Tuberculosis
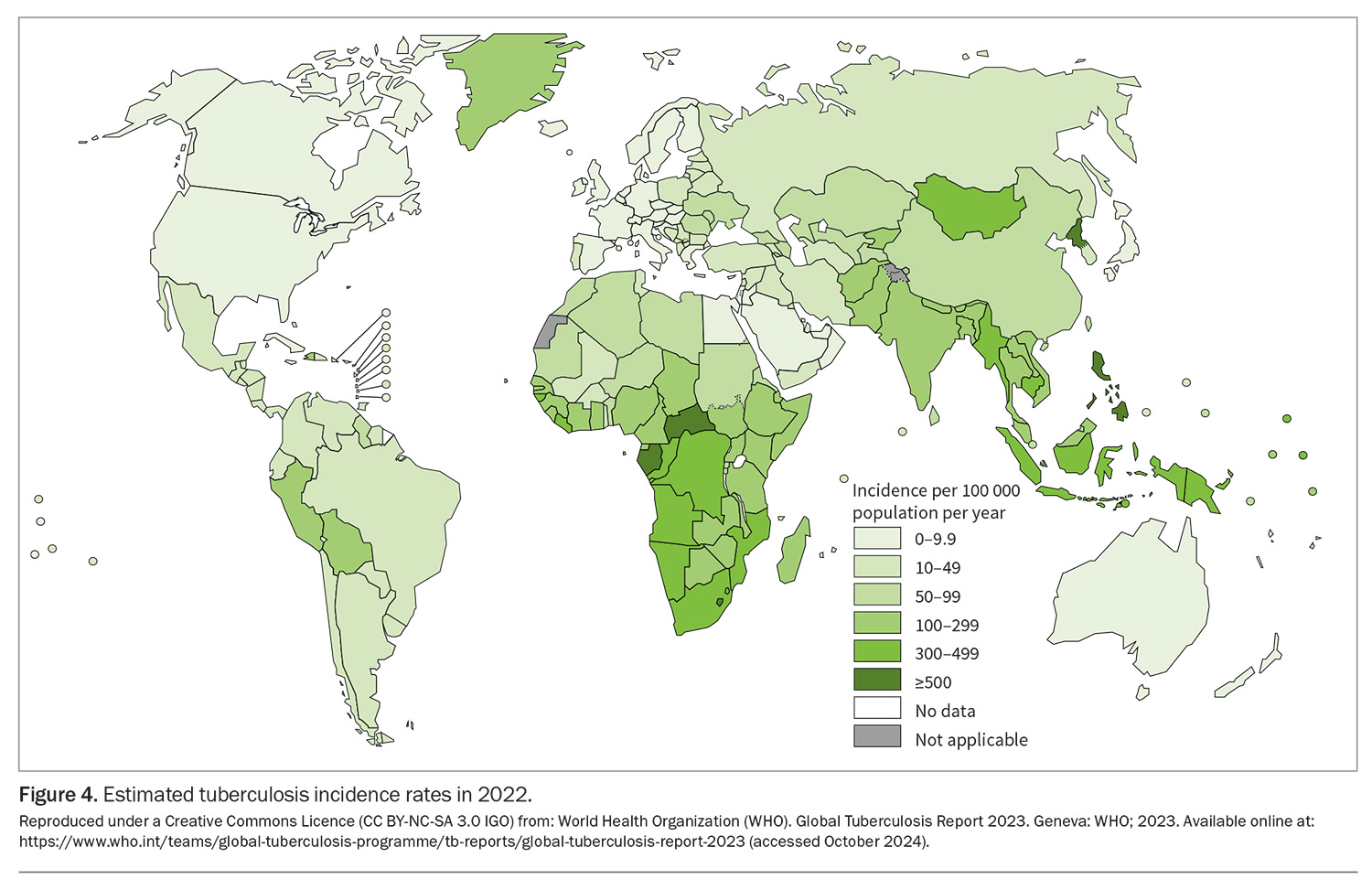
Young children travelling to countries with a high incidence of TB (more than 40 cases per 100,000 population per year) for an extended period (more than four weeks) are at increased risk of contracting TB and developing severe disease.3,22 Therefore, consider Bacille Calmette-Guérin (BCG) vaccination for any child aged younger than than 5 years travelling to a region of high tuberculosis prevalence for more than four weeks, or any child aged younger than 5 years visiting friends and relatives in one or more high TB prevalence countries for any period (Figure 4). In the authors’ clinics, these include families visiting friends and relatives in, but not limited to, India, Bangladesh, Pakistan, China, Brazil, Indonesia, Thailand, the Philippines and South Africa.
The only existing vaccine against TB, the BCG vaccine, which was created in 1921, has variable protective efficacy.23 As BCG vaccination does not prevent primary infection per se, nor does it prevent reactivation among those already infected with TB (e.g. latent TB), its role in preventing overall transmission is limited.23 However, there is strong evidence that BCG vaccination in childhood (before 5 years of age) provides at least 70 to 90% protection against severe disseminated forms of TB disease, including miliary and meningeal TB. However, this protection declines with increasing age.24 As TB can be difficult to diagnose in young children, and progression to disseminated disease can be rapid, they are the primary target for the use of the BCG vaccine. Vaccinating older children and adults appears to have less benefit. Nevertheless, consider vaccinating tuberculin-negative children aged 5 years to less than 16 years who may be living or travelling for long periods in high-risk countries.3 Identifying pregnant women whose infants may benefit from maternal BCG vaccination during antenatal care may also be helpful.
For child travellers who require the BCG vaccine, the following precautions should be considered when scheduling their vaccination visits. First, the BCG vaccine should preferably be given at least three months before entry into endemic areas. However, as this is rarely practical for people returning to visit their families, it should still be offered at any time before departure. Other injectable live viral vaccines (Box 2) should be administered either concurrently or with a minimum four-week interval before or after BCG vaccination.3 Second, tuberculin skin testing (Mantoux test) performed by a trained and accredited healthcare practitioner, is recommended by the NHMRC before administering the BCG vaccine for all individuals except infants aged younger than 6 months.3
In the authors’ practice, tuberculin testing is performed in children aged older than 2 years, as the majority of children younger than this age are Australian-born. However, if the child has a history of previous travel to a high TB-risk country or has lived with a known case of TB, tuberculin testing is performed, even if the child is aged younger than 2 years.
The response to tuberculin testing may be depressed for as long as four weeks after measles infection or administration of the MMR vaccine, and those interpreting the results of the tuberculin test should be aware of any other recently administered live injectable vaccines.3,25
Dengue
The dengue virus is transmitted to humans through the bite of an infected Aedes spp. mosquito, predominantly Aedes aegypti or Aedes albopictus. These mosquito species also transmit Chikungunya and Zika viruses. The best way to protect against dengue and other arboviral diseases is through mosquito bite avoidance measures.
Most Australian travellers are not recommended to receive dengue vaccine for short stays in dengue-endemic regions, even if they have previously had dengue, because of the risk-benefit balance. Although not registered in Australia, a dengue vaccine (Qdenga) is now available under the Special Access Scheme for the prevention of secondary dengue virus infection (not primary prevention of initial infection) in selected individuals. The UK Joint Committee on Vaccination and Immunisation (JCVI) recently released their deliberations on Qdenga, which is licensed in the United Kingdom for the use in dengue-seropositive individuals aged older than 4 years.26 The JCVI recommends that this vaccine can be offered to travellers who have evidence of previous dengue infection and are planning to regularly visit family or friends, or have been working or living abroad in high-burden endemic areas.26
Qdenga is a live attenuated vaccine and is therefore contraindicated in people who are immunosuppressed, immunocomprised, pregnant or breastfeeding. The vaccine is given as two doses, three months apart. The vaccine’s effectiveness against confirmed dengue is estimated to be higher in seropositive compared with seronegative individuals (64.2% vs 53.5%).27 The strict criteria and high cost (about $595 per dose) mean few individuals are suitable for this vaccine.
Mpox
Mpox, formerly known as monkeypox, is a newly emerging viral infection that has spread worldwide through mass gathering and travel. Mpox is caused by a virus in the Orthopoxvirus genus, which also includes the variola virus (which causes smallpox) and vaccinia virus (which is used in smallpox vaccines). There are two types of mpox virus: clade I and clade II. Clade II is considered to cause the less severe form of mpox and is the strain responsible for the global outbreak of mpox since May 2022, with over 102,000 reported cases from multiple countries where the illness is not usually seen, including Australia. In Australia, there were 144 case notifications in 2022, 26 in 2023 and, to date, 724 in 2024.14
The infection is usually self-limiting, with most people recovering within a few weeks. However, severe illness can occur, particularly in immunocompromised individuals.
More recently, since 2023, there has been a separate upsurge of mpox cases affecting the Democratic Republic of the Congo, which relates to a new strain: clade Ib. This strain has rapidly spread to a growing number of African countries. On 14 August 2024, the WHO declared the upsurge of mpox cases a public health emergency of international concern. To date, there have been no reported clade I mpox cases in Australia.28
Anyone can contract mpox, but global data show higher levels of transmission within the sexual networks of men who have sex with men.28 A shared clinical decision- making approach to vaccination with smallpox vaccine is appropriate, based on a joint assessment of individual risks, benefits and vaccine availability.
Tick-borne encephalitis
Tick-borne encephalitis is caused by a tick-borne flavirus and is prevalent in areas in temperate regions of central and northern Europe. Hiking or camping in forested areas of endemic regions during the summer months increases a traveller’s risk. Safe and effective vaccines are available and recommended only for travellers at high risk of exposure. A tick-borne encephalitis vaccine is not registered in Australia but is available under the Special Access Scheme.
Conclusion
It is essential for practitioners to have a sound knowledge of the geographical, climate, cultural and other conditions that pose a risk to individual travellers, to alert the travellers to these risks and to inform them how to avoid or minimise harm. This must be done in a manner that does not frighten or discourage the traveller. A thorough pretravel preparation and medical consultation can mitigate avoidable health and safety risks.
An initial risk assessment needs to be undertaken to tailor the discussion to the relevant risks identified for the traveller. This must take the form of a conversation with the traveller, as all travellers will have varying levels of risk acceptance. The Travel Medicine Clinical Guidelines Australia and New Zealand, which are being developed by the Australasian College of Tropical Medicine, contain resources that practitioners may find useful to help support these conversations (https://www.travelmedicineguidelinesanz.org/index.php). The appropriate interventions can then be chosen based on the discussion and risks. The adage ‘if you can’t afford travel insurance, you can’t afford to travel’ should be discussed with every traveller.
Practitioners should be aware that vaccination costs can add up to as much as the traveller’s plane fare. Discretion is the byword: practitioners must consider the risks and consequences of a severe anaphylactic or adverse reaction to vaccination. Yet, they must also recognise that giving a vaccination may save the person’s life. MT
COMPETING INTERESTS: Dr Streeton is a Member of the Travel Health Advisory Group (THAG) as the RACP representative; has received consulting fees from Biocelect; and has stock or stock options with GSK and CSL. Dr Chu has received honoraria from the Immunisation Coalition for presentations and the chairing of meetings at educational events; received honoraria from BioCSL Seqirus, Sanofi, GSK and Moderna for lectures and presentations; received support from ACTM Australasian College of Tropical Medicine to attend the Southern Cross Travel Medicine Conference; has been a Member of Advisory Board for the Immunisation Coalition; and has participated in an Advisory Board for CSL Seqirus, GSK and Sanofi.
References
1. Steffen R, Behrens RH, Hill DR, Greenaway C, Leder K. Vaccine‐preventable travel health risks: what is the evidence–what are the gaps? J Travel Med 2015; 22: 1-12.
2. Steffen R, Chen LH, Leggat PA. Travel vaccines-priorities determined by incidence and impact. J Travel Med 2023; 30: taad085.
3. Australian Technical Advisory Group on Immunisation (ATAGI). Australian immunisation handbook. Canberra: Australian Government Department of Health; 2022. Available online at: https://immunisationhandbook.health.gov.au (accessed October 2024).
4. Streeton CL, Zwar N. Risk of exposure to hepatitis B and other blood-borne viruses among Australians who travel abroad. J Travel Med 2006; 13: 345-350.
5. World Health Organization. International health regulations (2005). 3rd ed. Geneva: WHO; 2016. Available online at: https://www.who.int/publications/i/item/9789241580496 (accessed October 2024).
6. US Centers for Disease Control and Prevention (CDC). Yellow fever maps. Atlanta, GA: CDC; 2019. Available online at: https://www.cdc.gov/yellowfever/maps/index.html (accessed October 2024).
7. US Centers for Disease Control and Prevention (CDC). CDC yellow book 2024. Health information for international travel. Atlanta, GA: CDC; 2024. Available online at: https://wwwnc.cdc.gov/travel/page/yellowbook-home (accessed October 2024).
8. World Health Organization. Travel advice. Vaccines. Geneva: WHO; 2018. Available online at: https://www.who.int/travel-advice/vaccines (accessed October 2024).
9. Gershman M, Staples JE. Yellow fever. In: CDC yellow book 2024. Health information for international travel. Washington: US Department of Health and Human Services; 2024. Available online at: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/yellow-fever (accessed October 2024).
10. US Centers for Disease Control and Prevention (CDC). Global polio eradication. Updated December 1, 2023. Atlanta, GA: CDC; 2023. Available online at: https://www.cdc.gov/polio/global-polio-eradication.html (accessed October 2024).
11. US Centers for Disease Control and Prevention (CDC). Travelers’ health. Global polio. Atlanta, GA: CDC; 2024. Available online at: https://wwwnc.cdc.gov/travel/notices/level2/global-polio (accessed October 2024).
12. US Centers for Disease Control and Prevention (CDC). Polio for Travellers. Available online at: https://www.cdc.gov/polio/us/travelers.html (accessed October 2024).
13. Jelinek T, Kollaritsch H. Vaccination with Dukoral against travelers’ diarrhea (ETEC) and cholera. Expert Rev Vaccines 2008; 7: 561-567.
14. National Notifiable Disease Surveillance System. National communicable disease surveillance dashboard. Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://nindss.health.gov.au/pbi-dashboard/ (accessed October 2024).
15. Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica serovar Paratyphi B. Clin Infect Dis 2007; 45 Suppl 1: S24-S28.
16. Hoad VC, Kiely P, Seed CR, Viennet E, Gosbell IB. An outbreak of Japanese encephalitis virus in Australia; what is the risk to blood safety? Viruses 2022; 14: 1935.
17. Lindquist L. Recent and historical trends in the epidemiology of Japanese encephalitis and its implication for risk assessment in travellers. J Travel Med 2018; 25 Suppl 1: S3-S9.
18. Lau CL, Mills DJ, Mayfield H, et al. A decision support tool for risk-benefit analysis of Japanese encephalitis vaccine in travellers. J Travel Med 2023; 30: taad113.
19. World Health Organization. Rabies vaccines: WHO position paper – April 2018. Weekly Epidemiol Rec 2018; 93: 201-220.
20. World Health Organization. Rabies. Key facts. 20 September 2023. Geneva: WHO; 2023. Available online at: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed October 2024).
21. National Centre for Immunisation Research and Surveillance (NCIRS). ATAGI recommendation for using 2 doses of currently approved rabies vaccines for rabies PrEP vaccination in people indicated to receive rabies PrEP vaccination. December 2023. Canberra: NCIRS; 2023. Available online at: https://ncirs.org.au (accessed October 2024).
22. Toms C, Stapledon R, Coulter C, Douglas P, National Tuberculosis Advisory Committee. Tuberculosis notifications in Australia, 2014. Commun Dis Intell Q Rep 2017; 41: E247-E263.
23. World Health Organization (WHO). BCG vaccine. Geneva: WHO; 2024. Available online at: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccines-quality/bcg (accessed October 2024).
24. Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58: 470-480.
25. Chapter 32: Tuberculosis. In: The Green Book. UK Health Security Agency, 2018. Available online at: https://assets.publishing.service.gov.uk/media/5b645a2140f0b66875559e93/_Greenbook_chapter_32_Tuberculosis_.pdf (accessed October 2024).
26. Joint Committee on Vaccination and Immunisation. Minute of the meeting held on 07 February 2024. Available online at: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/1477553418407 (accessed October 2024).
27. Joint Committee on Vaccination snd Immunisation. Draft minutes, February 2024. London. Available online at: https://app.box.com/s/iddfb4ppwkmtjusir2tc/ file/1477553418407 (accessed October 2024).
28. ATAGI interim statement on the use of vaccines for prevention of mpox in 2024. 29 August 2024. Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://www.health.gov.au/resources/publications/atagi-interim-statement-on-the-use-of-vaccines-for-prevention-of-mpox-in-2024?language=en (accessed October 2024).