Depression: a major challenge of the menopause transition

Women are more likely to develop depressive symptoms during the perimenopause compared with other periods of their life, including women with no previous history of depression. Guidelines recommend antidepressant medications as first-line treatment; however, emerging evidence suggests menopausal hormone treatment may also be effective. A biopsychosocial approach to management, including treating depressive symptoms and addressing relevant psychological and lifestyle factors, offers the best outcomes and improvement in quality of life.
- The onset of menopause can trigger symptoms of depression in women at midlife, including recurrent and new-onset depression.
- The early association between perimenopause and the onset of depressive symptoms is often missed because of the lag between the earlier onset of symptoms of depression, anxiety and cognitive changes in perimenopause and the later onset of more obvious vasomotor symptoms.
- Routine gonadal hormone peripheral blood tests do not measure the brain fluctuations in hormones – hence blood tests do not assist in the diagnosis of menopausal depression.
- Careful history taking, including for risk factors for menopause-related depression, and assessment should be done to rule out other causes of depressive symptoms.
- A woman’s subjective assessment of her symptoms should be taken into consideration, particularly observations of a sudden change in mental health with no clear precipitating factors in her environment.
- Although traditional antidepressants are the recommended first-line treatment for depression in perimenopause, menopausal hormone treatment may also be effective alone or in combination with antidepressants.
- A biopsychosocial approach to management that incorporates tailored pharmacological treatments alongside lifestyle changes optimises outcomes.
Depressive symptoms are common in women aged in their mid 40s to early 50s. Women at midlife (45 to 54 years of age) have the highest rates of recurrent depression of all women, and women with no previous history of depression in this age group are two to four times more likely to experience depression in the menopause transition compared with younger and older women.1-4 Women in this important middle part of life face a number of challenges that have major psychological impacts, including concerns about ageing, dealing with workplace issues, being parents of adolescents, caring for elderly relatives and interpersonal challenges in their intimate relationships. However, in women at midlife, another important factor in the development of first-time depression or exacerbation of previous depression is the onset of menopause. Although most women do not experience significant mental ill health during the transition to menopause, an estimated 20% of perimenopausal women present to their primary health physician with depressive symptoms that may not be recognised as specifically related to menopause.5
Often, depressive symptoms experienced in the menopause transition are of a greater severity compared with before and after menopause.6 Importantly, and not coincidentally, suicide rates for women in the US were highest among those aged 45 to 64 years in both 2000 (6.2 per 100,000 women) and 2016 (9.9 per 100,000).7 Similarly, the highest completed suicide rates in women in Australia in 2021 were in those aged 45 to 49 years.8 Therefore, it is important for clinicians to understand, recognise and manage the different types of depression that can occur in women at midlife, and which may well be due to menopausal changes. Psychiatric hospitalisation may be needed for women whose depressive symptoms, especially suicidality, have become persistent and overwhelming.
Menopausal transition
The perimenopause marks the transition from a woman’s reproductive stage to menopause. Usually occurring between the ages of 42 and 52 years, perimenopause is determined clinically by the onset of irregular menstrual cycles or variable cycle lengths. According to the Stages of Reproductive Age Workshop (STRAW), cycle lengths must differ by at least seven days and, after a woman has had one year without a menstrual period, she has completed the transition into menopause.9 In addition to numerous somatic symptoms, one in three women will experience significant psychological changes during the transition into menopause.4
Studies show that women, including those with no previous history of depression, are more likely to develop depressive symptoms during the perimenopause compared with other periods of their life.4,10,11 It has been reported that hot flushes accentuate the risk of depressive symptoms in perimenopause;4,12 however, many high quality studies have shown an increased risk of having depression in the menopausal transition, even when controlled for hot flushes and life stressors.13,14 There is now crucial evidence showing that in menopause, the major gonadal hormone fluctuations impact higher brain functions, resulting in depression and related anxiety as well as cognitive changes in women who are particularly sensitive to the impact of hormone fluctuations.15,16
Neurobiology of menopausal depression
The neurobiology of depression is not fully understood but is thought, at least in part, to involve dopamine-serotonin pathways, with many dopamine pathways modulated by serotonergic neurons.17 Models of depression involve altered mesolimbic signalling and dysfunction of amygdala circuits involved in emotional control.18,19 Symptoms of depression are reversed with medications that increase dopaminergic and serotonergic transmission, indicating that depression is associated with a decline in serotonin and dopamine.20,21
Oestrogen has been shown to impact serotonin transmission by modulating serotonin receptor expression.22 Oestrogen levels fluctuate during menopause, particularly the perimenopause, causing destabilising effects on mood, possibly due to changes in serotonergic neurotransmission.23,24 Changes in transmission of other major neuropeptides, such as dehydroepiandrosterone sulphate (DHEAS) and gamma-aminobutyric acid (GABA), may be associated with depression in menopause. Levels of DHEAS decline with age, and a relationship between lower levels of DHEAS in older women and increasing symptoms of depression has been described.15,25 Similarly, a decline in GABAergic inhibitory function is seen in postmenopausal depression and parallels the reduced levels of GABA described in major depression models.26,27
Regardless of the downstream neurobiological changes in the key neurotransmitter systems seen in depression, the role of oestrogen as a neuroprotective agent is important, since it appears that the fluctuation and decline in brain oestrogen levels are a crucial factor in the development of menopausal depression.
Oestrogen affects brain function
Oestrogen continually contributes to brain growth and development by regulating cell survival, differentiation, proliferation and migration, and is ultimately involved in neurogenesis.22,28,29 Oestrogen is essential to maintaining growth factors, with oestrogen decline associated with reduced levels of brain-derived neurotropic factor.30 Animal and human studies have shown that oestrogen improves the activity of neuronal antioxidants, thereby protecting against neurodegenerative diseases.22,31,32 Oestrogen also affects glucose metabolism in the brain by augmenting glucose transporters, which enables better glucose utilisation, a process paramount to neuronal homeostasis.33,34 Moreover, estradiol-treated patients have reduced hippocampal atrophy, preserved age-related loss of grey matter and improved cerebral blood flow.35-37
Overall, the positive effects of oestrogen on brain structure, function and integrity – independent of age – are clear, thus, menopause may be a time of higher risk for brain disorders. Women exhibit increasing rates of amyloid deposition, accelerated hippocampal volume loss and reduced glucose utilisation across the menopausal transition, particularly within the early stage of perimenopause to menopause.16,38
Neuroscience provides some basis for understanding the significant impact of menopausal gonadal hormone fluctuations in the central nervous system and subsequent development of depression and related mental illness. A wealth of data from animal and clinical studies show broad beneficial effects of oestrogen administration for the brain, and hence mental health.
Diagnosing menopause-related depression is difficult
Despite growing evidence that menopausal gonadal hormone fluctuations impact the brain and subsequent development of depression and related mental illness, the use of gonadal hormone therapy in mental illness is rare and, to date, seen as experimental. This is in part due to the difficulty in recognising and diagnosing menopause-related depression. There are several reasons for this.
Women often experience symptoms of depression and associated anxiety and cognitive changes in early perimenopause, well before the easy-to-recognise vasomotor symptoms. Therefore, the link between perimenopause and the onset of depressive symptoms is often missed.
Menopause-related hormone changes are often missed as a key factor for depressive symptoms among the other psychosocial challenges that many women in their mid 40s face.
There is no specific test to detect menopausal depression. Routine blood tests are unable to detect the fluctuations in central nervous system oestrogens and precursors and their impact on brain chemistry. As a result, normal hormone levels are seen in perimenopausal or postmenopausal women with depression.39 Peripheral blood tests of the hypothalamic-pituitary-gonadal axis show changes in late transition to menopause – when clinical vasomotor symptoms are already obvious.
The current staging of menopause does not address the onset of mental health changes as the first symptoms of perimenopause in most women.40
Symptoms of menopause-related depression fluctuate
There appear to be two groups of women with menopause-related depression. The first is women with relapse depression, in whom previously treated depression re-presents, often with new added symptoms, and does not respond to previous or standard antidepressant treatments. The second group is de novo depression in women with no history of mental illness.
Some distinguishing features of menopause-related depression can be ascertained on clinical interviewing. Women in their mid-40s are well aware of their ‘usual’ stresses and coping styles, therefore, their subjective assessment of and views on their symptoms are crucial. Clinicians should take note of a woman’s observations of a sudden change in her mental health with no clear precipitating factors in her environment.
It is not unusual for a woman to be profoundly depressed for days, then feel totally well for a week, only to plunge back into depression. This is due to the underlying fluctuations in the hypothalamic-pituitary-gonadal axis hormones, resulting in the fluctuating nature of menopausal depression. This is not bipolar disorder and it is important to resist this diagnosis, as such a diagnosis is usually followed by the prescription of many mood stabilisers and antipsychotics – which create unwanted physical and mental side effects.
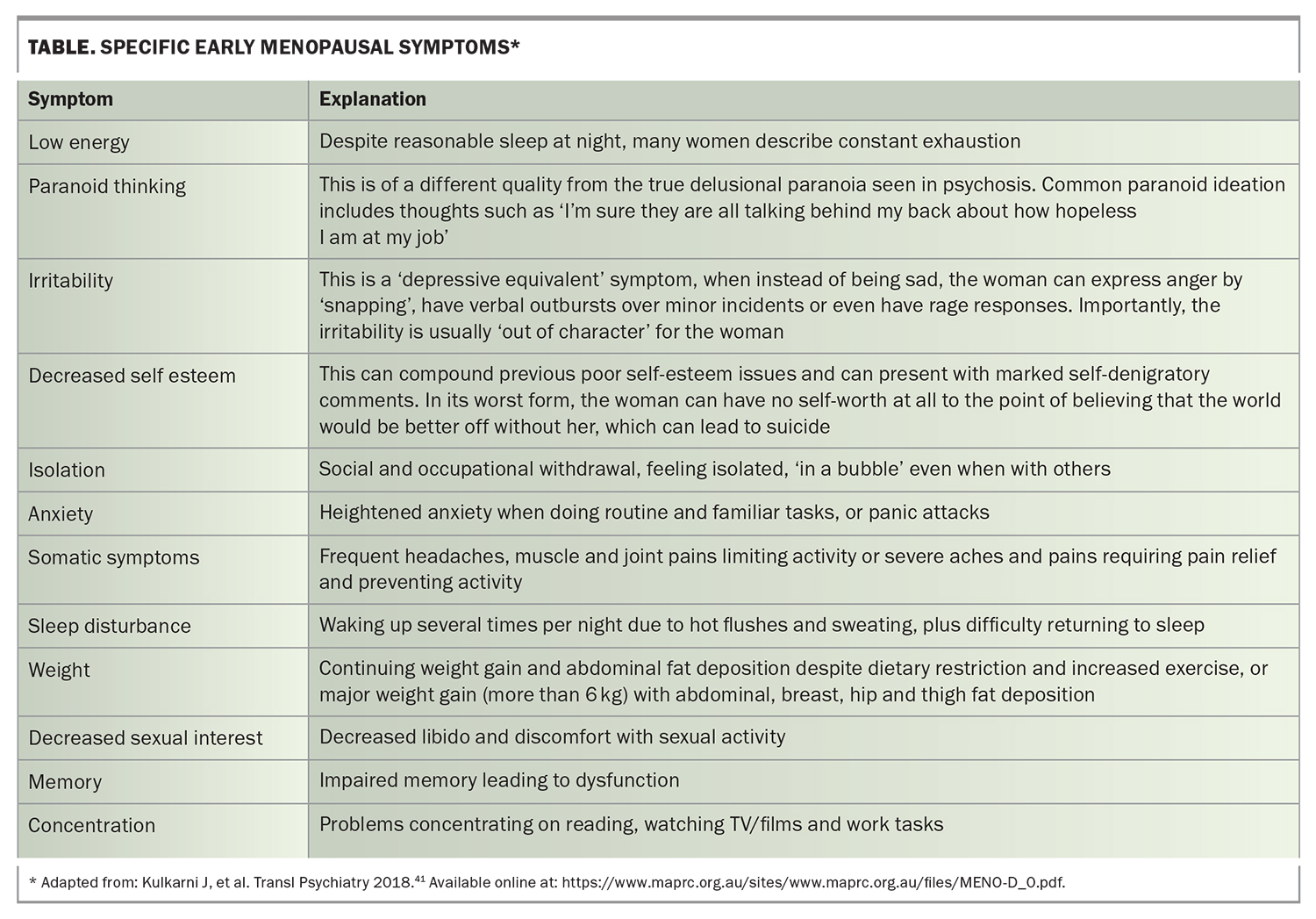
To aid the clinician, a validated questionnaire called the ‘MENO- D’ has been developed for use in any clinical practice setting.41 Specific symptoms to look for in early menopause are summarised in the Table.
Risk factors for developing menopause-related depression
Some women appear to be at greater risk of developing menopause-related depression. It is helpful to consider these risk factors as part of a clinical history and assessment of women with menopause hormone fluctuations, to delineate if symptoms are a part of the aetiology. Hormonal factors that appear to predispose women for menopause-related depression include:
- a history of premenstrual dysphoric disorder (PMDD)
- a history of depressive response to hormone contraceptives
- a history of depressive response to fertility hormone treatments
- a history of early and later life emotional, physical or sexual trauma, which create changes in the hypothalamic-pituitary-adrenal axis42
- severe vasomotor symptoms
- recent cessation of hormone contraception, especially if it has been taken for more than five years
- use of prolactin-elevating medications (e.g. some antipsychotics).
Perpetuating factors for any depressive disorder include past mental health problems (especially depression), excessive alcohol use, illicit drug use, poor diet, little or no exercise, poor physical health, poor support from friends and family, financial stressors, a high burden of care for others and workplace stresses.43
Investigations for menopause-related depression
After taking a clinical history (perhaps using the MENO- D questionnaire) and performing both a physical and mental state examination, it is important to consider the following relevant investigations to support or rule out a diagnosis of menopausal depression.
- An important differential diagnosis is hypothyroidism; therefore, thyroid function tests should be performed to confirm or rule this out.44 It is important to continue to monitor patients for hypothyroidism because thyroid disease is an independent risk factor for depression in menopausal women.
- Tests to rule out anaemia and vitamin D and B12 deficiency, which are associated with depressive symptoms.
- Other investigations relevant to all menopausal women include the following.
- A dual-energy x-ray absorptiometry scan is indicated as an important health measure to evaluate bone mineral density in postmenopausal women.44
- Since the risk of cardiovascular disease rises after menopause, an ECG and blood lipids profile are useful baseline tests.
- A mammogram and breast ultrasound should be considered for women aged 50 years and over. Women aged under 50 years may have a breast ultrasound if hormone treatment is being considered.
- Cervical screening tests need to be tailored to the woman’s age, human papillomavirus vaccination status and previous test results.45
- Although current guidelines do not recommend routine measurement of follicle-stimulating hormone (FSH) and luteinising hormone levels to assess menopausal status of women within the expected age range of the menopause transition, they can be measured under special circumstances, such as if menopause staging is needed. An FSH level higher than 40 IU/L can be used as a marker of final menopausal changes.40
It is important to remember that a woman may begin to notice changes in her mental state well before laboratory values reflect the changes and it is crucial that her observations are considered when planning treatment options.
Managing menopause-related depression
A holistic (biopsychosocial) and tailored approach that includes pharmacological treatments as well as lifestyle modifications is important to managing patients with mental health conditions. In treating menopause-related depression, input from a multidisciplinary team, including primary health physicians, gynaecologists, endocrinologists, psychologists and psychiatrists, may be needed to optimise outcomes for each woman.
Antidepressants and perimenopausal depression
Current guidelines recommend antidepressant medications, psychological therapy and lifestyle changes as first-line management for depression during menopause.18,46 These are not necessarily the best first-line treatments; rather, the recommendations reflect the paucity of clinical trials for hormone treatment in menopausal depression.
Antidepressants may not be efficacious for every woman, and many women describe a sense of increased emotional numbing when taking certain antidepressants and find this impairs their sense of living life fully.47 It is therefore important to tailor the choice of antidepressant for each woman. Commercially available pharmacogenomic testing can assist clinicians to choose an appropriate antidepressant and dose. Selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) are generally safe and effective but can have associated adverse effects such as serotonin syndrome, agitation, nausea, diarrhoea, anorexia, excessive sweating, decreased libido or anorgasmia, headache, insomnia and akathisia. Some postmenopausal women with major depressive disorder may not respond to the widely used SSRI escitalopram, and it is possible for older women to develop tachyphylaxis to SSRIs.19,48 Fluoxetine can have an agitating side effect and women with prominent insomnia, irritability and anxiety may report an exacerbation of these symptoms with fluoxetine treatment.49
The SNRI desvenlafaxine is the only antidepressant that has been studied for the treatment of perimenopausal depression in participants with clearly defined menopausal status. The randomised placebo-controlled study found that desvenlafaxine 50 mg daily was significantly efficacious in treating major depressive disorder in perimenopausal and postmenopausal women.50
Agomelatine is a newer antidepressant that has been shown to be effective in treating perimenopausal depression with minimal side effects.51 A particular advantage is in the positive impact that agomelatine treatment has on insomnia, which troubles many perimenopausal women.
Psychosocial treatment
Psychotherapy is an important intervention for women with perimenopausal depression that is clearly related to employment or relationship issues. A psychologist or GP with mental health expertise can provide required supportive or exploratory therapy. Other useful psychosocial approaches include discussions about regular exercise, mindfulness techniques, yoga and providing dietary advice. Be aware that many women in early transition experience increased anxiety and use readily available alcohol for self-medication. Minimising alcohol use is very important for improving mental state and reducing physical health impacts.
Menopausal hormone treatment (MHT)
Hormone therapy alone may be appropriate for middle-aged women with mild depression, without suicidality and who are able to have MHT, when the doctor is confident that symptoms are suggestive of menopausal changes. Contraindications for MHT are:
- oral oestrogen therapy in women with personal history of venous thromboembolism
- current or recent breast cancer, or other hormone-dependent cancers.
Breast cancer risk should be evaluated before MHT is prescribed. The risk of breast cancer associated with MHT in women aged over 50 years is complex, with increased risk primarily associated with the addition of a synthetic progestogen to estrogen therapy (conjugated equine estrogen [CEE] and medroxyprogesterone acetate [MPA] continuous combined therapy) and related to the duration of use.52
Emerging approaches for effective MHT include starting early, careful selection of patients, personalising the dose and type to the patient and prescribing a low or ultra-low dose. The choice of MHT includes oestrogens and progestogens in different types and doses. The Australian Menopause Society (AMS) provide comprehensive evidence-based practice guidelines, updated in April 2022, that should be consulted when treating patients with MHT.53,54 It is important that MHT is part of a holistic management strategy that also includes lifestyle recommendations on diet, exercise, smoking and alcohol use for maintaining the health of women at midlife.
Currently, clinical trials of MHT in women with menopausal depression are limited. Overall, the data so far suggest that MHT is useful in treating perimenopausal but not postmenopausal depression. One large four-year study in recently postmenopausal women reported that CEE (0.45 mg/day, with cyclic progesterone), but not transdermal estradiol (0.05 mg/day, with cyclic progesterone), improved depressive symptoms when compared with placebo.55 Another large four-month trial found no effect with CEE (0.625 mg/day, with continuous MPA).56
One small study in perimenopausal women showed that depression improved significantly after three weeks’ treatment with transdermal estradiol (0.05 mg/day) compared with placebo.57 Another study showed that depressive disorders were significantly more likely to remit after 12 weeks of treatment with transdermal estradiol (0.1 mg/day) compared with placebo.58
MHT for menopausal depression: practice suggestions
Many types of progestins and oestrogens are available for MHT. However, to minimise the gonadal fluctuations that adversely impact on brain neurotransmitter systems, when using MHT to treat menopausal depression it is important to choose a ‘nondepressive’ progestin, an oestrogen that efficiently crosses the blood brain barrier and a more constant MHT regimen.
The combined oestrogen–progestogen oral contraceptive pills (COCs) are useful first-line treatments in early transition to menopause; however, many COCs are associated with increased depression.59 It may be useful to use a ‘mood-neutral’ pill for early menopause and a combined estradiol and nomegestrol pill has shown some promise in improving depressive symptoms in premenstrual dysphoric disorder and early menopausal depression.60
As a woman progresses in her menopausal transition, the COC may not sufficiently ease her symptoms. As the next MHT for menopausal depression, tibolone is a convenient synthetic steroid with a mixed hormonal profile. It has been shown to relieve climacteric symptoms, improve libido and assist in the management of perimenopausal anxiety and mild depression.61-64 Additionally, tibolone does not cause increased breast density but some women may experience intermenstrual bleeding with treatment.61
If tibolone treatment is not sufficient to ease symptoms, a next step in MHT for menopausal depression could be using a combination of a progestin and a more potent oestrogen, namely 17-beta estradiol, which effectively crosses the blood-brain barrier. There are many ways of delivering estradiol, and 50 mcg (medium dose) or 100 mcg (high dose) patches are commonly used. Pump delivery of estradiol is also a convenient delivery system. It is important not to use conjugated estrogen treatment for menopausal depression since these preparations do not cross into the brain. In addition to the estradiol, micronised oral progesterone (100 mg or 200 mg) is less depressive and can be administered as a regular regimen, even in women who have had a hysterectomy.54
Selective estrogen receptor modulators (SERMs) are a new class of synthetic hormones shown to have the beneficial therapeutic effects of oestrogen on bone, lipids and the brain while minimising adverse effects on the uterus and breasts.65,66 A newer combination of the SERM bazedoxifene with conjugated estrogens has been shown to improve menopausal symptoms in healthy postmenopausal women.67 It is commercially available for moderate to severe vasomotor symptoms associated with menopause in women with a uterus, and it has potential as a future option for treating menopausal depression; however, clinical trials with SERMs to treat depression are limited and more data on this aspect is needed.
The risks and benefits of MHT differ for women during the menopause transition compared with those for older postmenopausal women. The International Menopause Society does not recommend bioidentical hormones because of standardisation and dosing issues.52 The International Menopause Society guidelines further recommend: ‘Women taking MHT should have at least an annual consultation to include a physical examination, update of medical and family history, relevant laboratory and imaging investigations, a discussion on lifestyle, and strategies to prevent or reduce chronic disease. There is currently no indication for increased mammographic or cervical smear screening’.52
Combination MHT and antidepressant therapy
In menopausal women with depression who do not respond to first-line treatment with either MHT or antidepressants, both classes of treatment may be combined.68 An optimal combination is transdermal estradiol, oral progesterone (100 mg) and an antidepressant with a low potential for agitation or emotional numbing side effects, such as agomelatine and vortioxetine. Brain stimulation techniques such as transcranial magnetic stimulation may also be efficacious in combination with MHT. In such situations, all medication and therapy adverse effects need to be monitored carefully.
Conclusion
The menopausal transition is a major biological event that all women experience. Early recognition of mental health issues related to the menopause and initiating biologically tailored treatment are essential to effective management. Most women with perimenopausal depression respond to treatment, and many current and emerging hormone treatments are available. Depression in midlife women requires a new approach with hormone treatments and appropriate antidepressant medication as well as psychological and lifestyle considerations. MT
COMPETING INTERESTS: Professor Kulkarni has received honoraria for educational presentations from Janssen, Servier and Lundbeck Pharmaceutical.
References
1. Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the national comorbidity survey. Am J Psychiatry 1994; 151: 979-986.
2. Tangen T, Mykletun A. Depression and anxiety through the climacteric period: an epidemiologic study (HUNT-II). J Psychosom Obstet Gynecol 2008; 29: 125-231.
3. Dennerstein L, Guthrie JR, Clark M, Lehert P, Henderson VW. A population-based study of depressed mood in middle-aged, Australian-born women. Menopause 2004; 11: 563-568.
4. Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition. Arch Gen Psychiatry 2006; 63: 385-390.
5. Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Women’s Midlife Health 2015; 1: 2.
6. Steinberg EM, Rubinow DR, Bartko JJ, et al. A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry 2008; 69: 973-980.
7. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief 2016; 241: 1-8.
8. Australian Bureau of Statistics (ABS). Causes of death, Australia 2020. Canberra, ABS; 2021. Available online at: https://www.abs.gov.au/statistics/health/causes-death/causes-death-australia/2020 (accessed September 2022).
9. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001; 76: 874-878.
10. Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry 2004; 61: 62-70.
11. Maartens LWF, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas 2002; 42: 195-200.
12. Reed SD, Ludman EJ, Newton KM, et al. Depressive symptoms and menopausal burden in the midlife. Maturitas 2009; 62: 306.
13. Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med 2011; 41: 1879-1888.
14. De Novaes Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001; 58: 529-534.
15. Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St. Clair LS. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology 2002; 27: 907-920.
16. Mosconi L, Berti V, Dyke J, et al. Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci Rep 2021; 11: 10867.
17. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64: 327-337.
18. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015; 100: 3975-4011.
19. Soares CN, Thase ME, Clayton A, et al. Open-label treatment with desvenlafaxine in postmenopausal women with major depressive disorder not responding to acute treatment with desvenlafaxine or escitalopram. CNS Drugs 2011; 25: 227-238.
20. Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 2008; 29: 683-695.
21. Jakobsen JC, Katakam KK, Schou A, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 2017; 17: 58.
22. Bustamante-Barrientos FA, Méndez-Ruette M, Ortloff A, et al. The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: beneficial or harmful? Front Cell Neurosci 2021; 15: 636176.
23. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006; 63: 375-382.
24. Halbreich U. Gonadal hormones, reproductive age, and women with depression. Arch Gen Psychiatry 2000; 57: 1163-1164.
25. Morrison MF, Ten Have T, Freeman EW, Sammel MD, Grisso JA. DHEA-S levels and depressive symptoms in a cohort of African American and Caucasian women in the late reproductive years. Biol Psychiatry 2001; 50: 705-711.
26. Wang Z, Zhang A, Zhao B, et al. GABA+ levels in postmenopausal women with mild-to-moderate depression. A preliminary study. Med (United States) 2016; 95: e4918.
27. Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 2010; 67: 458-464.
28. Pinceti E, Shults CL, Rao YS, Pak TR. Differential effects of E2 on MAPK activity in the brain and heart of aged female rats. PLoS One 2016; 11: e0160276.
29. McClure RES, Barha CK, Galea LAM. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav 2013; 63: 144-157.
30. Chhibber A, Woody S, Rumi MAK, Soares M, Liqin Z. Estrogen receptor β deficiency impairs BDNF-5-HT 2A signaling in the hippocampus of female brain: a possible mechanism for menopausal depression. Psychoneuroendocrinology 2017; 82: 107-116.
31. Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and amino- transferases in naturally menopausal rats. Exp Gerontol 2005; 40: 295-302.
32. Bellanti F, Matteo M, Rollo T, et al. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol 2013; 1: 340-346.
33. Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J 2001; 15: 907-915.
34. Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage 2004; 21: 364-371.
35. Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging 2003; 24: 725-732.
36. Boccardi M, Ghidoni R, Govoni S, et al. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a voxel-based morphometry study. Menopause 2006; 13: 584-591.
37. Cacciatore B, Paakkari I, Toivonen J, Tikkanen M, Ylikorkala O. Randomized comparison of oral and transdermal hormone replacement on carotid and uterine artery resistance to blood flow. Obstet Gynecol 1998; 92(4 Pt 1): 563-568.
38. Mosconi L, Rahman A, Diaz I, et al. Increased Alzheimer’s risk during the menopause transition: a 3-year longitudinal brain imaging study. PLoS One 2018; 13: e0207885.
39. Charney DA, Stewart DE. Psychiatric aspects. In: A Clinician’s Guide to Menopause. Steward DE, Robinson GE, eds. Washington, DC: Health Press International; 1997. Pp 129-144.
40. Harlow SD, Gass M, Hall JE, et al for the STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012; 19: 387-395.
41. Kulkarni J, Gavrilidis E, Hudaib AR, et al. Development and validation of a new rating scale for perimenopausal depression - the Meno-D. Transl Psychiatry 2018; 8: 123.
42. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs 2018; 32: 179-187.
43. Dennerstein L, Lehert P, Burger H, Dudley E. Mood and the menopausal transition. J Nerv Ment Dis 1999; 187: 685-691.
44. Gramann SB, Lundquist RS, Langenfeld SC, Talavera F, Memon M. Menopause and mood disorders. Ahmed I (ed). Medscape 2012.
45. Department of health and aged care. National Cervical Screening Program. Canberra, Department of Health and Aged Care: 2022. Available online at: https://www.health.gov.au/initiatives-and-programs/national-cervical-screening-program (accessed September 2022).
46. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Managing menopausal symptoms 2020. Available online at: https://ranzcog.edu.au/wp-content/uploads/2022/05/Managing-menopausal-symptoms.pdf (accessed September 2022).
47. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry 2021; 12: 792960.
48. Grigoriadis S, Kennedy SH, Bagby RM. A comparison of antidepressant response in younger and older women. J Clin Psychopharmacol 2003; 23: 405-407.
49. Kornstein SG, Jiang Q, Reddy S, Musgnung JJ, Guico-Pabia CJ. Short-term efficacy and safety of desvenlafaxine in a randomized, placebo-controlled study of perimenopausal and postmenopausal women with major depressive disorder. J Clin Psychiatry 2010; 71: 1088-1096.
50. Kulkarni J. Perimenopausal depression – an under-recognised entity. Aust Prescr 2018; 41: 183-185.
51. Kruger S, Tran T. EPA-1061 Agomelatine in the treatment of perimenopausal depression - a pilot study. Eur Psychiatry 2014; 29 Suppl 1: 1.
52. RJ Baber, N Panay, A Fenton; IMS Writing Group 2016. IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19: 109-150.
53. Australian Menopause Society. Menopause treatment options. AMS, Victoria; 2018. Available online at: https://www.menopause.org.au/hp/management/treatment-options (accessed September 2022).
54. Australian Menopause Society. AMS guide to equivalent MHT/HRT doses Australia only. AMS, Victoria; 2022. Available online at: https://www.menopause.org.au/hp/information-sheets/ams-guide-to-equivalent-mht-hrt-doses (accessed September 2022).
55. Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med 2015; 12: e1001833.
56. Maki PM, Gast MJ, Vieweg A, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology 2007; 69: 1322-1330.
57. Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000; 183: 414-420.
58. Soares CD, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001; 58: 529-534.
59. Skovlund CW, Mørch LC, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry 2016; 73: 1154-1162.
60. Robertson E, Thew C, Thomas N, Karimi L, Kulkarni J. Pilot data on the feasibility and clinical outcomes of a nomegestrol acetate oral contraceptive pill in women with premenstrual dysphoric disorder. Front Endocrinol (Lausanne) 2021; 12: 704488.
61. Van der Vies J. Pharmacological studies with (7 alpha,17 alpha)-17-hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one (Org OD 14). Maturitas 1987; 1: 15-24.
62. Karsidag AYK, KarsidagğC, Büyükbayrak EE, et al. Effects of tibolone on depressive and anxiety symptoms in symptomatic postmenopausal women. J Psychiat Neurol Sci 2012; 25: 135-139.
63. Khan N, Gavrilidis E, Kulkarni J. Tibolone treatment for perimenopausal depression: three cases. Aust N Z J Psychiatry 2016; 50: 1213-1214.
64. Kulkarni J, Gavrilidis E, Thomas N, et al. Tibolone improves depression in women through the menopause transition: a double-blind randomized controlled trial of adjunctive tibolone. J Affect Disord 2018; 236: 88-92.
65. Littleton-Kearney MT, Ostrowski NL, Cox DA, Rossberg MI, Hurn PD. Selective estrogen receptor modulators: tissue actions and potential for CNS protection. CNS Drug Rev Fall 2002; 8: 309-330.
66. Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther 2000; 295: 431-437.
67. Lello S, Capozzi A, Scambia G. The tissue-selective estrogen complex (bazedoxifene/conjugated estrogens) for the treatment of menopause. Int J Endocrinol 2017; 2017: 5064725.
68. Parry BL. Optimal management of perimenopausal depression. Int J Womens Health 2010; 2: 143-151.