Vericiguat: a new weapon in the heart failure arsenal

Vericiguat was recently PBS-listed as a first-in-class agent to treat selected patients with chronic heart failure and reduced ejection fraction. Vericiguat expands the therapeutic options for patients and prescribers and offers new hope to reduce the burden of heart failure in high-risk patients.
An estimated 1 to 2% of Australians are affected by heart failure and about half of these have a left ventricular ejection fraction (EF) of less than 40%.1 In 2020-2021, heart failure was implicated in about 179,000 hospital admissions, and 26,000 deaths were caused by, or associated with, heart failure.2 These statistics highlight the sobering burden of morbidity and mortality carried by those with heart failure and the significant demand for healthcare spending, particularly in the context of recurrent hospitalisations and the frequent need for high acuity care.
Fortunately, a swathe of clinical trials in recent years have supported a range of new pharmacotherapies that have shown incremental benefits in reducing hospitalisations and deaths for patients with heart failure and reduced EF (HFrEF).3-5 These are reflected in the recently updated Australian consensus guidelines, which offer a clear, stepwise management algorithm.6 Indeed, it is incumbent on all cardiologists and general medical practitioners to be familiar with the range of therapeutic options available and how to tailor and optimise these for individuals, so as to best lessen the burden of disease for patients, their carers and the healthcare system.
This article focuses on a recently PBS-listed agent, vericiguat, and its particular role in managing high-risk patients with HFrEF who have been stabilised after an episode of decompensation. It briefly outlines the mechanism of action of vericiguat, examines the evidence for its use in an appropriately selected patient cohort, and offers practical tips on how to commence, monitor and adjust therapy.
How does vericiguat work?
Vericiguat belongs to a novel class of oral medications known as soluble guanylate cyclase (sGC) stimulators. These agents mimic and enhance the effect of nitric oxide in the circulatory system, activating a cellular cascade that results in relaxation of vascular smooth muscle and vasodilation.7 The promotion of nitric oxide signalling is thought to improve overall cardiac function through multiple mechanisms, directly increasing coronary artery blood flow and dampening vascular inflammation. This leads to positive remodelling of the ventricle by enhancing relaxation and reducing maladaptive changes such as myocardial hypertrophy and fibrosis.8,9 These pathways are also targeted by nitrates and phosphodiesterase (PDE)-5 inhibitors such as sildenafil, which have become part of established therapy for pulmonary arterial hypertension.
Riociguat, another sGC stimulator, has shown benefit in the treatment of pulmonary hypertension related to chronic thromboembolic disease.10 In the setting of heart failure, vericiguat is thought to improve renal blood flow and to promote the excretion of sodium and water, thereby counteracting the feedback mechanisms that would otherwise lead to fluid retention.11
What does the evidence say?
The use of vericiguat in patients with chronic heart failure and reduced EF is supported by the findings of a landmark clinical trial, VICTORIA (Vericiguat Global Study in Subjects with HFrEF), published in 2020.12 This multinational study enrolled 5050 adult patients experiencing symptomatic heart failure with an EF less than 45% who had been hospitalised in the preceding six months or received intravenous diuretics in the preceding three months for decompensated heart failure. These patients were randomised to receive vericiguat or placebo starting at 2.5 mg daily, with the dose progressively increased to 10 mg daily. After a median follow-up period of almost 11 months, it was found that patients who had received vericiguat were about 10% less likely either to die from a cardiovascular cause or to need hospitalisation for management of heart failure. This translates to treating about 24 patients with vericiguat for one year to prevent one cardiovascular death or heart failure admission.
In the VICTORIA trial, the benefits of vericiguat occurred within about three months of starting therapy and were driven by a reduction in the rate of heart failure hospitalisations. When the rate of cardiovascular deaths was examined in isolation, there was no significant difference between vericiguat and placebo. Patients aged under 75 years, those with EF less than 40% and those with New York Heart Association (NYHA) functional class III or IV symptoms tended to benefit most from vericiguat.
Surprisingly, patients with the highest levels of a biochemical indicator of more severe heart failure, N-terminal pro B-type natriuretic peptide (more than 5314 ng/L), tended to have worse outcomes with vericiguat. The exact reasons for this are unclear, but it perhaps reflects a subgroup of patients with very advanced disease and, likely, more complex comorbidities.
It is also worth noting that VICTORIA was conducted at a time when relatively few patients had been prescribed angiotensin receptor neprolysin inhibitors (ARNIs) or sodium-glucose cotransporter-2 (SGLT-2) inhibitors, as these agents were not yet established as components of standard heart failure therapy. The added benefit of vericiguat in patients receiving these agents is not clearly known. Longer term longitudinal studies are also awaited to assess whether the benefits of vericiguat are sustained beyond the follow-up period in the VICTORIA trial.
Who should receive vericiguat?
On 1 December 2022, vericiguat was added to the PBS general schedule for adults with chronic heart failure who have a confirmed left ventricular EF less than 45% and symptoms consistent with NYHA functional class II to IV (or at least mild limitation of ordinary physical activity).13 Vericiguat is PBS-listed for commencement in patients following a recent episode of decompensated heart failure that has required intravenous diuretics in the preceding three months or hospitalisation in the preceding six months. Patients must be stabilised and relatively euvolaemic when starting therapy, with systolic blood pressure measuring at least 100 mmHg and no requirement for intravenous therapy for fluid overload in the preceding 24 hours.
Vericiguat should be used only as add-on therapy for patients with persistent heart failure on top of maximally tolerated standard medical therapy, which must include, unless contraindicated or not tolerated, a beta blocker (carvedilol, bisoprolol, metoprolol, nebivolol) plus an ACE inhibitor, angiotensin II receptor blocker (ARB) or ARB-ARNI combination (sacubitril-valsartan). A mineralocorticoid receptor antagonist (MRA) and an SGLT-2 inhibitor are also recommended as part of standard therapy before the addition of vericiguat is considered.
Cardiac resynchronisation device therapy should also be considered in selected patients. Following this, vericiguat should be prescribed as adjunctive therapy in those felt to still be at high risk of hospital readmission.
It is important to bear in mind that the VICTORIA trial excluded patients with various cardiovascular conditions, including recent acute coronary syndromes, transient ischaemic attack or established stroke in the preceding 60 days, as well as those with acute myocarditis, primary valvular heart disease (requiring surgery or intervention), hypertrophic cardiomyopathy and infiltrative conditions such as amyloidosis and sarcoidosis.12 As such, the use of vericiguat cannot be clearly recommended in these patients.
Vericiguat can be used for patients with chronic kidney disease.14 However, it should be avoided in patients on dialysis or those with an estimated glomerular filtration rate less than 15 mL/min/1.73 m2. It should also be avoided in patients with severe liver dysfunction and encephalopathy.
Vericiguat is contraindicated in patients who are already taking long-acting nitrates, PDE-5 inhibitors or other sGC stimulators, as these patients were excluded from the VICTORIA trial and would be at increased theoretical risk of developing symptomatic hypotension.15 It is also contraindicated in the setting of current or anticipated pregnancy, as some early animal studies have found evidence of harm to the developing fetus.15
How should vericiguat be started?
Vericiguat may be commenced by a cardiologist or any medical practitioner who has been requested to do so in consultation with a cardiologist. The recommended starting dose is 2.5 mg daily, which should be gradually uptitrated every two weeks as tolerated, up to a target dose of 10 mg daily.15 In the VICTORIA trial, for instance, the vericiguat dose was doubled every two weeks as long as the patient's systolic blood pressure was 100 mmHg or higher. The available formulations are 2.5 mg, 5 mg and 10 mg tablets, with packs containing 28 tablets each. Vericiguat is best taken with food to enhance gut absorption.16
No dosing adjustments are generally required in the absence of severe renal or liver impairment, and the potential for significant drug interactions with vericiguat is considered to be low overall.16 Similar starting doses can be used in patients of advanced age, although clinical judgement should be applied, as always, in this vulnerable population.
What should I look out for?
Vericiguat has a generally robust safety profile across a range of doses in patients with recent decompensated heart failure and EF less than 45%. In the VICTORIA trial, adverse events were reported in 80.5% of patients taking vericiguat, but this rate was actually higher at 81% for patients assigned to placebo.12 Serious adverse events were also less common overall in patients taking vericiguat compared with placebo. Side effects that occurred more often, albeit only slightly, in those who received vericiguat versus placebo included hypotension (15.4% vs 14.1%), anaemia (7.6% vs 5.7%), dizziness (6.7% vs 6.0%), syncope (4.0% vs 3.5%), nausea (3.8% vs 2.7%) and headache (3.4% vs 2.4%). There were no clear safety concerns with respect to worsening renal function or electrolyte disturbances with vericiguat.14
In the VICTORIA trial, systolic blood pressure dropped modestly by less than 5 mmHg over the first four weeks of therapy but then returned to baseline after about eight months. Symptomatic hypotension occurred in 9.1% of patients taking vericiguat, versus 7.9% taking placebo; this difference was not statistically significant.12 Before reducing or ceasing vericiguat, ensure that other potential causes of hypotension are addressed and consider adjusting the doses of concurrent diuretics that have no prognostic benefit.
Overall, 89.2% of patients assigned to vericiguat in the VICTORIA trial were able to reach the target dose of 10 mg daily after about 12 months.12 This was similar to 91.4% in the placebo group, suggesting that vericiguat is reasonably well tolerated by most patients with heart failure.
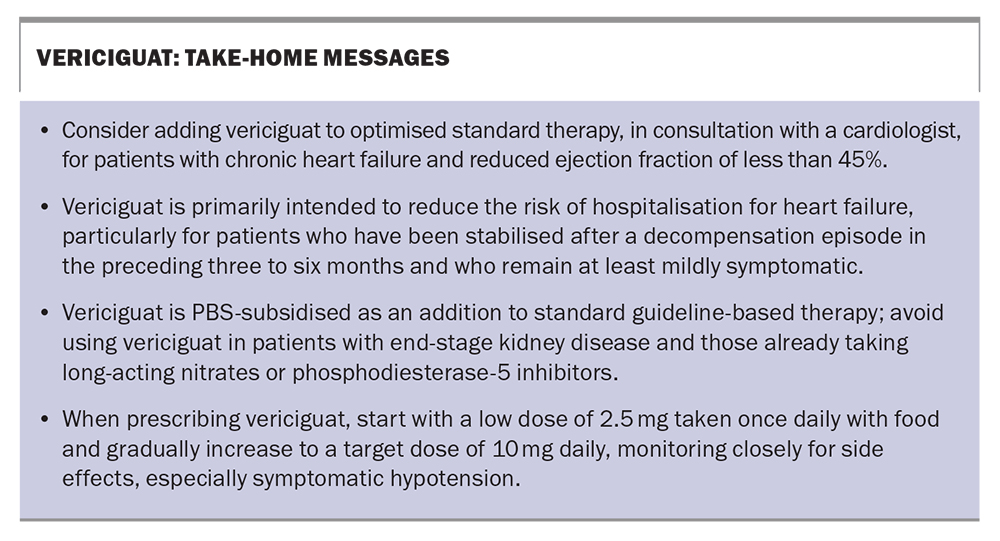
Take-home messages about vericiguat are shown in the Box.
Conclusion
The new sGC stimulator, vericiguat, was recently PBS-listed as add-on therapy for selected patients with symptomatic chronic heart failure with EF less than 45%. Patient selection criteria include stabilisation after a recent episode of decompensated heart failure that required intravenous diuretic therapy or hospitalisation. There is evidence that vericiguat reduces the need for rehospitalisation for heart failure. It may be commenced by a cardiologist, or any medical practitioner in consultation with a cardiologist. MT
COMPETING INTERESTS: Dr Wong: None. Associate Professor Muthiah has received speaker fees from Bayer.
References
1. Sahle BW, Owen AJ, Mutowo MP, Krum H, Reid CM. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord 2016; 16: 32.
2. Australian Institute of Health and Welfare (AIHW). Heart, stroke and vascular disease: Australian facts. Heart failure and cardiomyopathy. Canberra: AIHW; 2023. Available online at: https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/hsvd-facts/contents/heart-stroke-and-vascular-disease-and-subtypes/heart-failure-and-cardiomyopathy (accessed July 2023).
3. Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction. Circulation 2021; 143: 326-336.
4. McMurray JJ V, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995-2008.
5. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993-1004.
6. Sindone AP, De Pasquale C, Amerena J, et al. Consensus statement on the current pharmacological prevention and management of heart failure. Med J Aust 2022; 217: 212-217.
7. Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011; 123: 2263-2273.
8. Hulot JS, Trochu JN, Donal E, et al. Vericiguat for the treatment of heart failure: mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin Pharmacother 2021; 22: 1847-1855.
9. Emdin M, Aimo A, Castiglione V, et al. Targeting cyclic guanosine monophosphate to treat heart failure: JACC review topic of the week. J Am Coll Cardiol 2020; 76: 1795-1807.
10. Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319-329.
11. Lombardi CM, Cimino G, Pagnesi M, et al. Vericiguat for heart failure with reduced ejection fraction. Curr Cardiol Rep 2021; 23: 144.
12. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883-1893.
13. Pharmaceutical Benefits Scheme. Schedule of pharmaceutical benefits (summary of changes effective 1 December 2022). Canberra: Australian Government Department of Health; 2022.
14. Voors AA, Mulder H, Reyes E, et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur J Heart Fail 2021; 23: 1313-1321.
15. Bayer Australia. Australian product information. Verquvo (vericiguat) film-coated tablets. Sydney: Bayer Australia; 2021.
16. Boettcher M, Gerisch M, Lobmeyer M, et al. Metabolism and pharmacokinetic drug–drug interaction profile of vericiguat, a soluble guanylate cyclase stimulator: results from preclinical and phase I healthy volunteer studies. Clin Pharmacokinet 2020; 59: 1407-1418.