Heart failure with preserved ejection fraction: advances in management
About 50% of heart failure presentations are due to heart failure with preserved ejection fraction (HFpEF). Compared with heart failure with reduced ejection fraction (HFrEF), HFpEF is a highly heterogeneous disease, and is associated with more comorbidities that do not gain mortality benefits from HFrEF therapies. However, recent advances, including the use of sodium-glucose cotransporter-2 inhibitors and patient phenotype profiling, are changing the management of HFpEF.
- Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are the first proven treatment for heart failure with preserved ejection fraction (HFpEF) demonstrated to reduce heart failure hospitalisation or cardiovascular death and, more importantly for patients, to improve symptoms and quality of life. SGLT-2 inhibitors should be used in all patients with HFpEF without contraindications.
- Profiling patient comorbidities assists in defining appropriately directed medical therapy for certain patient groups, which may not have otherwise proved efficacious in trials of this heterogeneous HFpEF patient group.
- Optimising management of contributory cardiovascular comorbidities is pertinent, and includes obesity management, control of hypertension and exercise programs to improve functional capacity.
- Spironolactone may be helpful in certain populations, and is a useful adjunct in patients with concomitant hypertension.
- Other medical therapies that have proven benefits in other cardiovascular populations, including glucagon-like peptide-1 receptor agonists and intravenous iron, are undergoing further investigation to define their role in the HFpEF population.
- Evidence is emerging for the use of implantable pulmonary artery pressure monitors to guide decongestion and avoid heart failure hospitalisations.
Heart failure with preserved ejection fraction (HFpEF) is estimated to affect 536,000 adults in Australia. It is a rapidly increasing problem, with a projected overall lifetime risk of around 20% at age 40 years.1,2 This increasing risk is driven by an aging population and an increasing burden of associated cardiometabolic risk factors, including hypertension, diabetes, chronic kidney disease (CKD), atrial fibrillation (AF), obesity and obstructive sleep apnoea (OSA). The challenges in diagnosing HFpEF and a practical approach to assessing patients who present with shortness of breath or oedema are addressed in an accompanying article.3 This article focuses on the management of HFpEF.
Pharmacological management of HFpEF
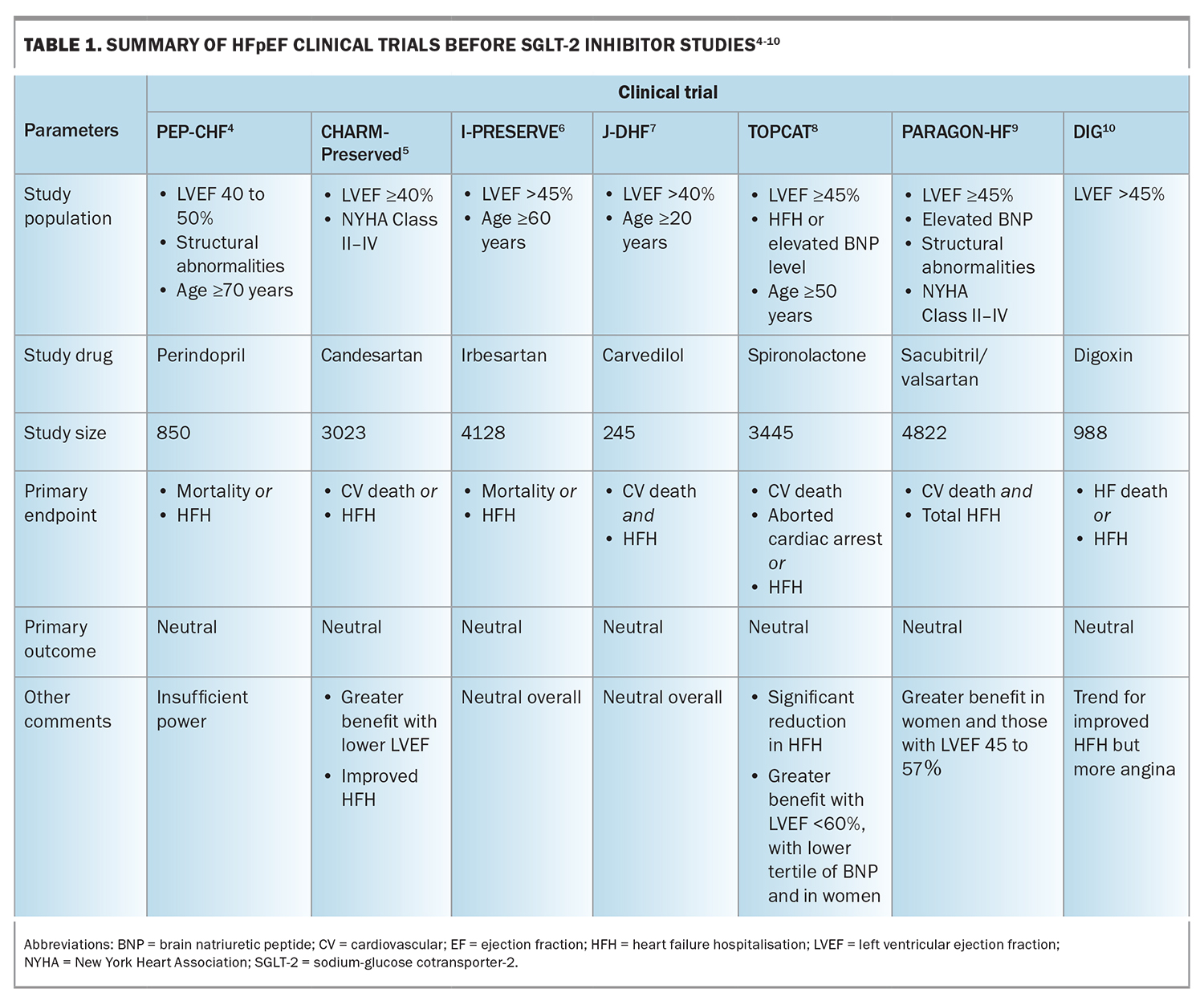
Guideline-directed medical therapy (GDMT) exists for the management of HFrEF; however, specific GDMT has been lacking for HFpEF. The management of HFpEF has, therefore, traditionally focused on alleviating symptoms of fluid overload using diuretics, and managing risk factors and associated comorbidities. Clinical trials using conventional HFrEF agents have shown no improvement in the outcomes of those with HFpEF (Table 1).4-10 However, the latest successful trials with sodium-glucose cotransporter-2 (SGLT-2) inhibitors herald the arrival of GDMT for the management of HFpEF.11,12
SGLT-2 inhibitors
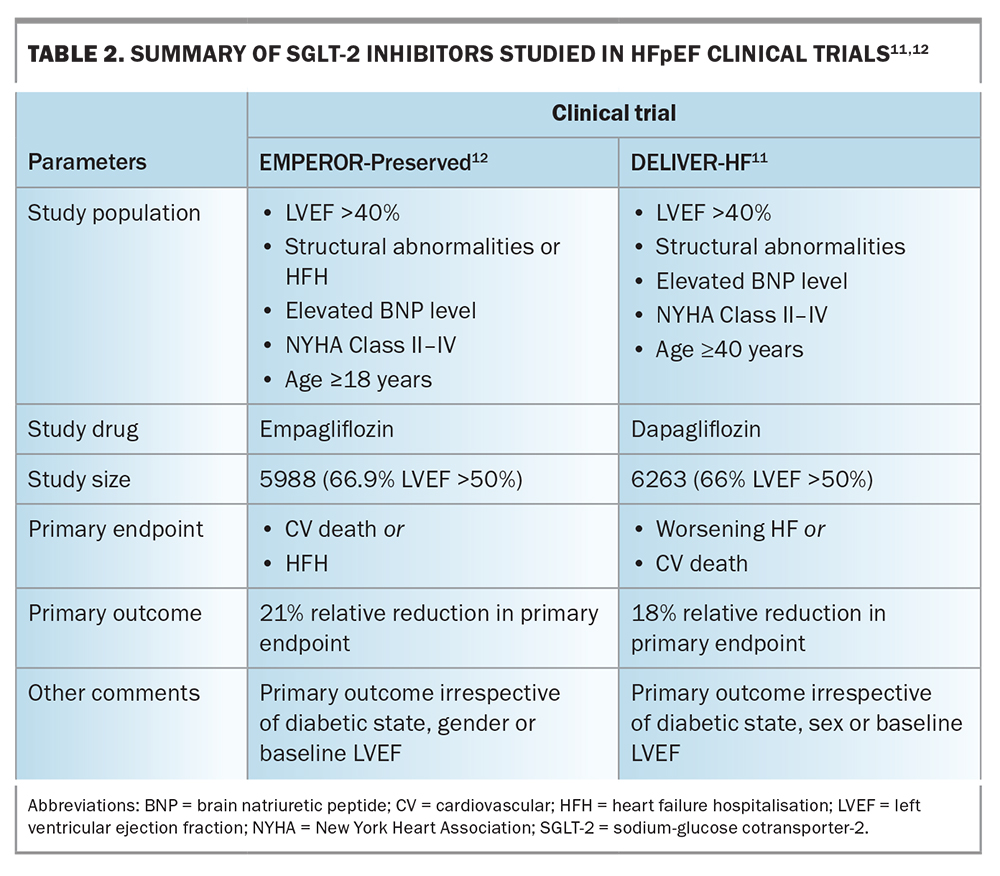
SGLT-2 inhibitors are the first class of medications demonstrated in adequately powered randomised controlled studies to reduce the combined endpoint of heart failure (HF) hospitalisation or cardiovascular mortality in HFpEF. The two major trials of SGLT-2 inhibitors in patients with HFpEF have demonstrated a significant reduction of about 20% in the risk of hospitalisation for HF and cardiovascular death in those taking dapagliflozin (DELIVER trial) or empagliflozin (EMPEROR-Preserved trial) compared with placebo (Table 2).11-13 The observed benefit of these drugs appeared to be additive to the existing treatments of mineralocorticoid antagonists (MRAs) and angiotensin receptor-neprilysin inhibitors (ARNIs), and was not influenced by the presence or absence of diabetes, baseline ejection fraction or sex.14 More important, from a patient’s perspective, were the significant improvements in symptoms, health-related quality of life and improved exertional tolerance.13 However, it is important to note that the benefits shown in the trials were driven by a reduction in hospitalisations rather than cardiovascular deaths.
Early introduction of the SGLT-2 inhibitor empagliflozin in the management of hospitalised patients during an acute decompensation episode has been shown to be safe and well tolerated, and observed benefits may be due to its diuretic effect.15 Furthermore, the outcome benefits were seen as early as 18 days after starting treatment.8 The main limitation of SGLT-2 inhibitor use is in patients with severe renal impairment, as empagliflozin requires an estimated glomerular filtration rate (eGFR) of
20 mL/min/1.73 m2 or higher and dapagliflozin an eGFR of 25 mL/min/1.73 m2 or higher at the time of initiation. However, SGLT-2 inhibitors have also been demonstrated to slow the progression of CKD.16-18 Therefore, although SGLT-2 inhibitors should not be commenced in patients with an eGFR below these ranges, there is no routine requirement to discontinue them if the eGFR falls below these cut-offs after commencement. Indeed, the eGFR is anticipated to decrease in the initial two to four weeks after commencement of an SGLT-2 inhibitor, but will usually recover.18 These highly compelling data have led to the first (and only) recommendation that all patients with HFpEF should be initiated on an SGLT-2 inhibitor in the absence of clear contraindications.19
Angiotensin receptor-neprilysin inhibitors
In the PARAGON-HF trial, patients with a left ventricular ejection fraction (LVEF) of 45% or higher, elevated natriuretic peptide levels and evidence of structural heart disease were randomised to either the ARNI sacubitril/valsartan at a target dose of 97/103 mg twice daily or valsartan alone (not placebo or angiotensin-converting enzyme [ACE] inhibitors). These patients had a numerically lower, but not statistically significant, benefit of reduced total hospitalisations for HF and cardiovascular death.9 Subsequent analysis showed greater benefit in those with an LVEF between 45 and 57%, and in women. Sacubitril/valsartan is approved for use in HFpEF in the US; however, there is no recommendation for such use in Australia.19
Mineralocorticoid antagonists
MRAs such as spironolactone have been shown to improve measures of diastolic dysfunction in patients with HFpEF.20 The TOPCAT trial evaluated spironolactone versus placebo in patients with HF and LVEF of 45% or higher, and showed a small but not statistically significant reduction in the composite endpoint of death, aborted cardiac death or HF hospitalisations.8 However, these results were confounded by limited adherence to therapy in one of the treatment regions, and subsequent analysis suggested a reduction in the risk of HF hospitalisation.21 Furthermore, benefits were shown for some subgroups, including women and those with an LVEF below 60% and a lower tertile of B-type natriuretic peptide. Therefore, spironolactone may be considered for use as an adjunct to the diuretic regimen, as well as for managing hypertension. Appropriate monitoring of the serum creatine and potassium levels is required at initiation and follow up due to the risk of worsening kidney function and hyperkalaemia.
Angiotensin receptor blockers
Trials with angiotensin receptor blockers (ARBs) have shown mixed results. A trial of candesartan in patients with an LVEF of 40% or higher demonstrated a borderline significant reduction in the primary composite outcome of HF hospitalisation or cardiovascular death, but a moderate reduction in the individual component of HF hospitalisations when compared with placebo.5 Trials of irbesartan have not demonstrated similar results.4 Current data suggest sacubitril/valsartan is likely to be more effective than an ARB, and is preferred in select patients.
Nonpharmacological management of HFpEF
Nonpharmacological management of HFpEF focuses mainly on strategies to optimise contributing comorbidities, including weight loss management and exercise, as well as a potential role for HF rehabilitation and emerging evidence for the use of device therapies.
Exercise and cardiac rehabilitation
Physical inactivity and obesity are strongly linked to an increased risk of HF, with worse health status and poorer prognosis in those with HFpEF.22 Exercise is key for improving functional capacity, and guidelines recommend more than 150 minutes/week of preferably aerobic physical activity to also assist with weight loss.23 Enrolment in cardiac rehabilitation programs, especially in patients with prior hospitalisation, may improve quality of life and functional capacity in patients with HFpEF.24 Exercise with a view to weight loss and other weight reduction strategies are discussed below.
Device therapies in HFpEF
Device therapies that have been explored in HFpEF include the use of implantable pulmonary artery pressure (PAP) monitors and interatrial shunt devices. The use of PAP monitors to detect early rises in PAP and guide decongestion management have been shown to decrease HF hospitalisations.25-27 Several interatrial shunt devices designed to create an internal shunt from the left to right atrium to prevent the rise in left atrial pressure with exercise, the hallmark and main driver of symptoms in HFpEF, have shown early promising results in small studies.28 Further larger studies are currently underway.
Patient phenotype profiling and management of comorbidities
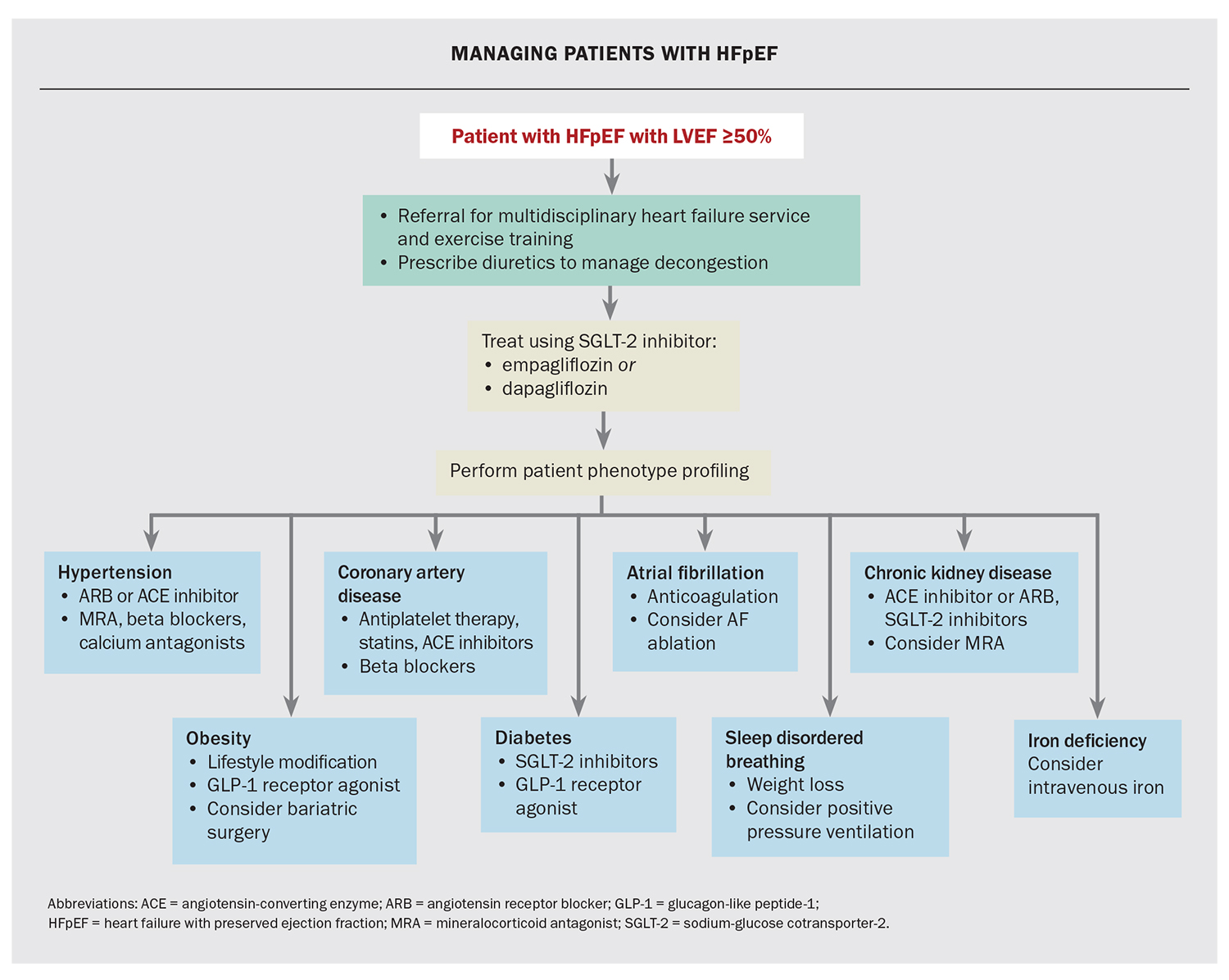
As HFpEF is a heterogeneous disease associated with variable comorbidities, it is important to identify these in order to tailor individual patient management. Some key comorbidities are discussed below (Flowchart).
Hypertension
Hypertension is highly prevalent in HFpEF, affecting 60% to 89% of the HFpEF population.29,30 The role of blood pressure control is well established for the prevention of HF as well as in reducing the risk of other cardiovascular events in these patients, who often have other associated cardiometabolic risk factors. Recent consensus from the American College of Cardiology recommends a systolic blood pressure of lower than 130 mmHg in patients with HFpEF and hypertension who do not have symptomatic orthostasis.30 Of note, blood pressure-lowering has not been associated with improved outcomes in those with already established HFpEF; however, uncontrolled blood pressure may precipitate acute HF decompensation.8 Choice of antihypertensive agents may be guided by tolerability, cost and comorbidities. Given the supporting data described previously, candesartan and spironolactone may be preferred agents. Beta blockers may be useful for patients with other indications, such as symptomatic coronary artery disease or atrial fibrillation with rapid ventricular response; however, they can contribute to chronotropic incompetence, which may further limit exercise tolerance in some patients.31,32
Obesity
Obesity is one of the strongest risk factors for developing HFpEF: body mass index is directly correlated with HF risk, and 80% of patients with HFpEF are either overweight or have obesity.32-34 An increasing severity of obesity correlates with an increased risk for HF-associated hospitalisation, as well as increased risk of other cardiometabolic risk factors, AF and OSA.32 Patients with HFpEF who are obese are younger and have poorer functional class.32 Studies suggest a beneficial effect of weight loss, either via caloric restriction or bariatric surgery, on incident HF events and exercise tolerance, and these pathways may be considered in the appropriate patient.35
Support for preventing obesity and encouraging weight loss through lifestyle modifications, such as diet and exercise, are recommended. Pharmacological management with newer agents, including the glucagon-like peptide-1 (GLP-1) receptor antagonist semaglutide and the combined GLP-1/glucose-dependent insulinotropic-polypeptide agonist tirzepatide, has been shown to be effective in achieving weight loss in patients with obesity, with improvement in quality of life, irrespective of diabetic status.36,37 A recent study in HFpEF populations showed significant benefits of semaglutide on patient symptoms and quality of life.38 Further studies are underway to investigate its impact on clinical outcomes.39 For those with a body mass index above 35 kg/m2, referral to a multidisciplinary specialised obesity clinic may be considered, including discussion of surgical options.40
Coronary artery disease
Epicardial coronary artery disease is present in over 50% of patients with HFpEF, which may also contribute to symptoms of dyspnoea.41 No prospective trials have evaluated the benefit of revascularisation on symptoms or mortality in people with HFpEF. Revascularisation should, therefore, be pursued according to standard practice guidelines, alongside aggressive secondary prevention and risk factor optimisation. The long-acting nitrate isosorbide mononitrate has been shown to decrease activity levels compared with placebo, with no measurable benefit and is, therefore, not routinely recommended in HFpEF.42
Diabetes
Diabetes is prevalent in people with HFpEF, affecting up to 40% of patients, and correlates with a twofold risk of associated HF hospitalisation and mortality compared with those without diabetes.43,44 Optimisation of diabetic control is recommended according to the Australian Evidence-Based Clinical Guidelines for Diabetes.45
Atrial fibrillation
AF and HF often coexist. AF has been shown to pertain to a worsened functional status and increased risk of hospitalisation, as well as mortality in those with HF.46 No clear benefit in a rhythm (rate vs rhythm) or rate (strict vs lenient) control strategy have been reported.47,48 An individualised approach is recommended and, as such, an attempt at achieving sinus rhythm with a rhythm control strategy may be beneficial to assess an associated improvement in symptoms. A small, open-label trial in older people with AF and HF suggested a benefit in functional capacity from digoxin for rate control at 12 months, which may be related to lower rates of dizziness, lethargy and hypotension, when compared with beta blockers.49 Aggressive rate control should be avoided because of the potential to reduce stroke volume.
Sleep disordered breathing/obstructive sleep apnoea
Sleep disordered breathing, of which OSA is the most common subtype, affects up to 55 to 80% of individuals with HFpEF, has adverse effects on quality of life and is associated with an increased risk of depression.50 Treatment of OSA has not been shown to have a definitive benefit on cardiovascular outcomes in HFpEF; however, smaller studies suggest positive improvements in both symptoms and objective measures of diastolic function and cardiovascular endpoints.51-54 There is evidence that treating OSA in patients with treatment-resistant hypertension may be beneficial in controlling hypertension, as well as have possible benefits in reducing the risk of recurrent AF; therefore, screening for OSA, particularly in these cohorts, remains important.55
Chronic kidney disease
CKD is present in about 50% of patients with HFpEF and is associated with an up to threefold increase in mortality.56,57 Patients with concomitant CKD and HFpEF will benefit from renin-angiotensin system inhibition in combination with an SGLT-2 inhibitor to delay decline in renal function.18,58 Therefore, the use of these agents is strongly encouraged in the absence of other contraindications.
Iron deficiency
Iron deficiency is present in 50 to 75% of patients with HFpEF, and appears to be more prevalent in these patients than in those with HF with mid-range ejection fraction or HFrEF.57,59 Iron deficiency in HFpEF is associated with worse symptoms and reduced exertional tolerance and overall quality of life.60 Although iron replacement has been associated with reduced HF hospitalisation in patients with HFrEF, no clear data are available for those with HFpEF.60-62 Trials are currently underway in patients with HFpEF (FAIR-HFpEF, PREFER-HF trials) to investigate the impact of intravenous iron versus placebo on exercise capacity.
Conclusion
HFpEF is common and results in poor quality of life, frequent hospitalisations and increased risk of death, as seen with HFrEF. SGLT-2 inhibitors are the first class of drugs recommended for all patients with HFpEF to improve symptoms and quality of life and reduce HF hospitalisations or cardiovascular death. Diuretics are recommended for the management of oedema or congestion. Due to the heterogeneity of symptoms and comorbidities in HFpEF, management of contributing comorbidities should be tailored to each patient. MT
COMPETING INTERESTS: Dr Jeffries: None. Dr Chan has received payment or honoraria from AstraZeneca, Biotronik, Boeringer-Ingelheim, Medtronic, Novartis, Pfizer Vifor; is a Board Member of the Heart Foundation; and is the SA Board Representative of the Cardiac Society of Australia and New Zealand.
References
1. Chen L, Booley S, Keates AK, Stewart S. Snapshot of heart failure in Australia. Mary MacKillop Institute for Health Research. 2017; Australian Catholic University, Melbourne. Available online at: https://www.acu.edu.au/about-acu/news/2017/june/snapshot-of-heart-failure-in-australia (accessed September 2023).
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106: 3068-3072.
3. Holland DJ, Blazak PL, Atherton JJ, Prasad S. Heart failure with preserved ejection fraction – an exclusive diagnosis. Med Today 2023: 24(1-2): 23-28.
4. Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27: 2338-2345.
5. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003; 362: 777-781.
6. Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2011; 4: 324-331.
7. Yamamoto K, Origasa H, Hori M; J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013; 15: 110-118.
8. Selvaraj S, Claggett B, Shah SJ, et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail 2018; 20: 483-490.
9. McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020; 141: 338-351.
10. Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation 2006; 114: 397-403.
11. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 287: 1089-1098.
12. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 285: 1451-1461.
13. Vaduganthan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised control trials. Lancet 2022; 400: 757-767.
14. Verma S, Dhingra NK, Butler J, et al. Empagliflozin in the treatment of heart failure with reduced ejection fraction in addition to background therapies and therapeutic combinations (EMPEROR-Reduced): a post-hoc analysis of a randomised, double-blind trial. Lancet Diabetes Endocrinol 2022; 10: 35-45.
15. Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalised for acute heart failure: a multinational randomised trial. Nat Med 2022; 28: 568-574.
16. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
17. Yang D, Zhang Y, Yan J, Liu M, An F. SGLT-2 inhibitors on prognosis and health-related quality of life in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. Front Cardiovasc Med 2022; 9: 942125.
18 Di Costanzo A, Esposito G, Indolfi C, Spaccarotella CAM. SGLT2 inhibitors: a new therapeutical strategy to improve clinical outcomes in patients with chronic kidney diseases. Int J Mol Sci 2023; 24: 8732.
19. Sindone AP, De Pasquale C, Amerena J, et al. Consensus statement on the current pharmacological prevention and management of heart failure. Med J Aust 2022; 217: 212-217.
20. Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the ALDO-DHF randomised controlled trial. JAMA 2013; 309: 781-791.
21. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl Med 2014; 370: 1383-1392.
22. Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation 2016; 133: 639-649.
23. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J AM Coll Cardiol 2019; 74: e177-e232.
24. Kizman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalised for heart failure. N Engl J Med 2021; 385: 203-216.
25. Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014; 7: 935-944.
26. Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658-666.
27. Brugts JJ, Radhoe SP, Clephas PRD; MONITOR-HF investigators. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial. Lancet 2023; 401: 2113-2123.
28 Feldman T, Mauri L, Kahwash R, et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [reduce elevated left atrial pressure in patients with heart failure]): a phase 2, randomized, sham-controlled trial. Circulation 2018; 137: 364-375.
29. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251-259.
30. Kittleson M, Panjrath G, Amancherla K, et al. 2023 ACC Expert consensus decision pathway on management of heart failure with preserved ejection fraction. J Am Coll Cardiol 2023; 81: 1835-1878.
31. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta blockers in heart failure: findings from the OPTIMIZE-HF (Organised Program to Initiate Lifesaving Treatment in Hospitalised Patients with Heart Failure) Registry. J Am Coll Cardiol 2009; 53: 184-192.
32. Mandviwala TKU, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker? Curr Atheroscler Rep 2016; 18: 21.
33. Khalid U, Wruck LM, Quiberera RM, et al. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: the Atherosclerosis Risk in Communities Surveillance Study. Int J Cardiol 2017; 233: 61-66.
34. Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 2016; 68: 200-203.
35. Persson CE, Bjorck L, Lagergren J, et al. Risk of heart failure in obese patients with and without bariatric surgery in Sweden-a registry-based study. J Card Fail 2017; 23: 530-537.
36. John PH, Wilding DM, Batterham RL, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989-1002.
37. Jastreboff A, Aronne LJ, Ahmad NN, Wharton S, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205-216.
38. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069-1084.
39. Novo Nordisc. Semaglutide 2.4 mg reduces the risk of major adverse cardiovascular events by 20% in adults with overweight or obesity in the SELECT trial. Company announcement, 8 August 2023. Available online at: https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=166301 (accessed September 2023).
40. Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for metabolic and bariatric surgery. Surg Obes Relat Dis 2022; 18: 1345-1356.
41. Rush CJ, Berry C, Oldroyd KG, et al. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021; 6: 1130-1143.
42. Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314-2324.
43. Anguilar D, Deswal A, Ramasubbu K, et al. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol 2010; 105: 373-377.
44. Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019; 140: e294-e324.
45. Living Evidence for Diabetes Consortium. Australian Evidence-Based Clinical Guidelines for Diabetes. 2020, Living Evidence for Diabetes Consortium. Available online at: https://www.diabetessociety.com.au/20211104%20Guideline-Australian-Evidence-Based-Clinical-Guidelines-for-Diabetes.pdf (accessed September 2023).
46. Zafrir B, Lung LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range and preserved ejection fraction: a report from 14964 patents in the European Society of Cardiology Heart Failure Long-term Registry. Eur Heart J 2018; 39: 4277-4284.
47. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. Eur J Heart Fail 2009; 11: 676-683.
48. Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362: 1363-1373.
49 Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomised clinical trial. JAMA 2020; 324: 2497-2508.
50. Gupta N, Agrawal S, Goel AD, et al. Profile of sleep disordered breathing in heart failure with preserved ejection fraction. Monaldi Arch Chest Dis 2020; 90.
51. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375: 919-931.
52. Yoshihisa A, Suzuki S, Yamaki T, et al. Beneficial effects of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail 2013; 15: 543-550.
53. O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69: 1577-1587.
54. Torres G, Sanchez-de-la-Torre M, Barbe F. Relationship between OSA and hypertension. Chest 2015; 148; 824-832.
55. Affas Z, Affas S, Tabbaa K. Continuous positive airway pressure reduces the incidence of atrial fibrillation in patients with obstructive sleep apnea: a meta-analysis and systematic review. Spartan Med Res J 2022; 7: 34521.
56. Vijay K, Neuen BL, Lerma EV. Heart failure in patients with diabetes and chronic kidney disease: challenges and opportunities. Cardiorenal Med 2022; 12: 1-10.
57. Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39372 patients from 30 studies. Eur Heart J 2013; 34: 1404-1413.
58. McCausland FR, Lefkowitz MP,Claggett B, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020; 142: 1236-1245.
59. Masini G, Graham FJ, Pellicori P, et al. Criteria for iron deficiency in patients with heart failure. J Am Coll Cardiol 2022; 79: 341-351.
60. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Open Heart 2019; 6: e001012.
61. Pezel T, Audureau E, Mansourati J, et al. Diagnosis and treatment of iron deficiency in heart failure: oFICSel study by the French heart failure working group. ESC Heart Fail 2021; 8: 1509-1521.
62. Ponikowski P, Mentz RJ, Hernandez AF, et al. Efficacy of ferric carboxymaltose in heart failure with iron deficiency: an individual patient data meta-analysis. Eur Heart J 2023; ehad586.